
Achievement of a primary clinical trial endpoint is essential for drug approval by regulatory bodies; therefore, the appropriate selection of a primary endpoint is not only critical for the success of an investigational therapy but also for patients who would benefit from treatment. This concept is echoed by the challenges – historical, current, and subsequent – of trial design for geographic atrophy (GA) interventions. GA is an advanced form of age-related macular degeneration (AMD) characterized by the progressive loss of retinal photoreceptors, the retinal pigment epithelium (RPE), and the underlying choriocapillaris, which can result in irreversible vision loss (1). In North America, GA accounts for 20 percent of legal blindness, with numbers expected to increase in the coming decades (2). Currently, several clinical trials are evaluating the efficacy and safety of various investigational compounds for GA, mostly in phase I or II (3). More recent phase III trials evaluating pegcetacoplan (OAKS and DERBY) and avacincaptad pegol (GATHER1 and GATHER2) have reported at least 12-month data in the past year, resulting in pegcetacoplan becoming the first FDA-approved treatment for GA in February 2023 and avacincaptad pegol becoming the second FDA-approved treatment in August 2023 (4,5,6,7,8,9).
Despite decades of GA clinical trial research, there is still a need for effective and safe treatments for GA (4,10). According to a systematic review of 53 GA clinical trials registered at ClinicalTrials.gov, large differences in study design characteristics were found (10), suggesting a need for better consensus and standardization of trial design for GA interventions to facilitate drug discovery. Furthermore, endpoints not only need to be statistically significant, but also clinically meaningful (11,12). The aforementioned systematic review also determined that GA lesion growth was the most common primary endpoint, with visual acuity (VA) most commonly designated as a secondary endpoint (10). One might reasonably ask: What can be more clinically meaningful than vision? Here, we examine the rationale for clinical endpoints used in GA clinical trials, discuss potential alternative endpoints, and explore where the future may take us.
Primary endpoint – why is VA the runner-up?
VA is a measure of central vision, and in GA patients, VA can be maintained earlier in the disease course, particularly if the fovea is spared from atrophy. It is not until GA progresses to include the fovea that severe and irreversible vision loss typically occurs (13,14,15,16). Furthermore, visual decline can be gradual and variable. One study demonstrated that a ≥three-line loss from baseline occurred by two years in 31 percent of patients with GA and by four years in 53 percent (17). Moreover, patients can adapt via eccentric fixation to optimize their VA (18). Therefore, it may not be deemed feasible to demonstrate a clinical benefit of a therapeutic intervention for GA using VA as a primary outcome measure in a clinical trial setting (19,20,21).
Determining how to measure changes in VA during a GA clinical trial is another challenge. Best corrected visual acuity (BCVA), or more generally, VA, has been the standard primary endpoint for most clinical trials in ophthalmology (21,22,23). In a trial setting, VA is typically measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, which consists of five-letter lines with every third line representing a doubling of the visual angle (22). The proportion of eyes losing or gaining ≥15 letters (≥three lines) has been a common primary outcome measure in wet AMD clinical trials, for example, and has resulted in several drug approvals (23,24). Many clinical trials evaluating interventions for GA that have met their primary endpoints did not demonstrate significant changes in VA, which was analyzed as the mean change in VA from baseline (9,25,26). A possible explanation could be a patient’s ability to engage viable islands of retinal tissue as a temporary compensatory mechanism, which would render substantial differences in mean change in VA difficult to demonstrate within the time frame of a clinical trial. As such, a categorical and time-to-event analysis of letter loss that accounts for the incidence of clinically significant vision loss may be a more appropriate method to characterize the patient journey of vision loss. This was recently reported for the GATHER1 and GATHER2 trials – despite not showing a statistically significant change in mean BCVA (27). Similarly, low-luminance VA (LLVA) has also been used in clinical trials, but also generally as a secondary endpoint (9,25,26,28). LLVA and low-luminance deficit (LLD) have been shown to be predictive of future BCVA loss, as LLVA may be affected earlier in GA progression than BCVA (29,30). In the ReCLAIM-2 clinical trial, LLVA was a co-primary endpoint, and although a change from baseline was not shown to be significant at Week 48 with elamipretide compared with placebo, a categorical two-line gain in LLVA was observed (p=0.04) (31).
The evolution of GA lesion growth as a primary endpoint
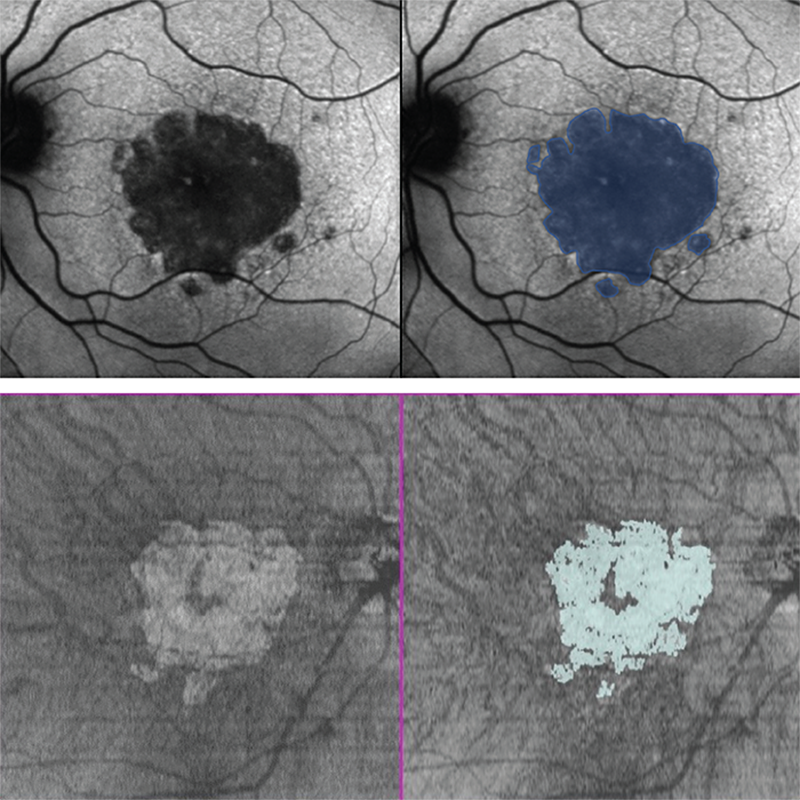
Ultimately, it is photoreceptor death that contributes to the irreversible vision loss associated with GA (1,32,33). Visual function is linked to the number of viable photoreceptors, which is the rationale for why GA growth – the most commonly used primary endpoint in both phase II and phase III GA trials – is used as a surrogate measure for VA (2,10,11,33,34). Further, as a structural endpoint, it is a reliable and objective measure that is not confounded by patient behavior (16). GA growth in clinical trials is frequently assessed using fundus autofluorescence (FAF) because its high-contrast visualization of atrophic regions facilitates accurate quantification of the lesion area (see Figure 1) (19,35). GA area can be measured either as observed (mm2) or square root transformed (mm), with the square root transformation often used in clinical trials to minimize the impact of baseline GA size, which has been shown to affect GA growth (21,36). However, the interpretation of GA as square root transformed may not be intuitive, as GA size is generally measured in square millimeters (mm2) in the clinic (36). A slope analysis of lesion growth is preferred by the FDA, which examines the mean rate of growth of the GA area over time, as assessed by FAF, from baseline to a particular time point and assumes a constant rate of growth over the period (37). The idea is that interventions that could significantly change the slope of GA growth would allow for monitoring of the trajectory of GA progression, and a reduction in GA growth may reflect photoreceptor preservation, which is necessary for maintaining vision (11,33,38,39,40). Therefore, several clinical trials have used slope analysis to assess GA growth (8,41,42).
New and up-and-coming clinical trial endpoints
Structural outcome measures are valuable because they are objective, reproducible, and obtained using imaging modalities common in the clinic; however, they often require validation from other imaging modalities, which can be complicated by the varying grading systems that define GA lesions (16,43). Optical coherence tomography (OCT) is a widely available diagnostic tool that allows the assessment and quantification of GA by measuring atrophy growth via en face visualization of the hypertransmission defects (see Figure 1) (1,35). Some studies have demonstrated the correlation between FAF and OCT measurements of GA lesions, although the borders of the lesion may not be identical with the two technologies (44,45). Another potential endpoint that can be evaluated on OCT is the progression from incomplete RPE and outer retinal atrophy (iRORA) to complete RPE and outer retinal atrophy (cRORA), which are OCT-based definitions of atrophy specified by the international Classification of Atrophy Meetings Group (CAM) (43). Post hoc analyses of the FILLY and GATHER1 studies have indicated a potential benefit with pegcetacoplan and avacincaptad pegol, respectively, in slowing the progression from iRORA to cRORA compared with untreated patients (46,47). The prevention of photoreceptor loss as seen on OCT represents an additional potential clinical trial endpoint. Photoreceptor degeneration and ellipsoid zone loss in eyes with GA have been explored with OCT, including in the ReCLAIM-2 clinical trial, and have been recommended as endpoints for clinical trials beyond GA lesion size growth (11,31,40). These types of analyses often require artificial intelligence/machine learning/deep learning (31,40) and are currently in their infancy but, in the future, such analyses could become routine endeavors.
Based on a National Eye Institute (NEI)/FDA endpoints workshop on AMD held in November 2016, functional endpoints (for example, visual field, contrast sensitivity, other light sensitivity measures) were preferred over anatomic endpoints, but structural endpoints should remain under consideration due to the variability in measuring visual function. The perspective of the European Medicines Agency (EMA) was largely aligned with that of the FDA. In principle, change in GA area could be acceptable as a primary efficacy variable in GA trials; however, it would be preferable if it was demonstrated together with an effect on visual function (11). By slowing or halting the progression of GA growth, the hope is that the foveal center point will not be affected, thus reducing vision loss and limiting negative effects on patients’ quality of life (QoL) (14,17,19,48). Although less frequently used in GA clinical trials at this time, functional measures, such as microperimetry and reading speed, are making their way into clinical trial programs, such as the Chroma, Spectri, and OAKS trials, and they offer measurements of function that are important to aspects of daily living (25,28). Quick quantitative contrast sensitivity function and dark adaptometry with shorter protocols could also be potential functional additions to future clinical trials (19,49-51). Other clinical endpoints that do not specifically evaluate structure or function but aim to assess QoL include the NEI Visual Function Questionnaire-25 and the Functional Reading Independence index score (16,25,28,48,52).
In brief
Current primary endpoints for GA clinical trials focus on the structural endpoint of GA lesion growth. Although improving vision or preventing visual impairment is the clinical benefit that eye care professionals aim for – and that patients seek – delaying critical tissue loss and reducing lesion growth may lead to delayed visual impairment and loss, which is still an integral step for a disease that has only recently seen the approval of its first two interventions.
Author Disclaimer
SriniVas Sadda: Consultant/Advisor: Iveric Bio; Biogen; Boehringer Ingelheim; 4DMT; Alexion; Allergan, Inc.; Alnylam Pharmaceuticals; Amgen Inc.; Apellis Pharmaceuticals, Inc.; Astellas; Bayer Healthcare Pharmaceuticals; Carl Zeiss Meditec; Catalyst Pharmaceuticals Inc.; CenterVue, Inc.; Genentech; Gyroscope Therapeutics; Heidelberg Engineering; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Nanoscope; RayTx; OTX; Novartis Pharma AG; Optos, Inc.; Oxurion/Thrombogenics; Pfizer Inc.; Regeneron Pharmaceuticals, Inc.; Samsung Bioepis; Vertex Pharmaceuticals Inc. Lecture Fees/Speakers Bureau: Carl Zeiss Meditec; Heidelberg Engineering; Nidek Inc.; Novartis Pharma AG; Topcon Medical Systems, Inc.; Roche/Genentech.
Medical writing support was provided by IMPRINT Science (New York, NY) and was funded by Iveric Bio, An Astellas Company.
References
- M Fleckenstein et al., “The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration,” Ophthalmology, 125, 369 (2018). PMID: 29110945.
- FG Holz et al., “Geographic atrophy: clinical features and potential therapeutic approaches,” Ophthalmology, 121, 1079 (2014). PMID: 24433969.
- Retina Specialist, “2023: The year of geographic atrophy” (2023). Available at: https://bit.ly/3re3GVs.
- Apellis, “FDA Approves SYFOVRE™ (pegcetacoplan injection) as the First and Only Treatment for Geographic Atrophy (GA), a Leading Cause of Blindness” (2023). Available at: https://bit.ly/3yfciLH.
- Astellas Pharma Inc., "Iveric Bio Receives U.S. FDA Approval for IZERVAYTM (avacincaptad pegol intravitreal solution), a New Treatment for Geographic Atrophy" (2023). Available at: https://bit.ly/3Qpq8Uf.
- N Steinle et al., “Efficacy of intravitreal pegcetacoplan in geographic atrophy: results from the DERBY and OAKS trials.” Presented at the 39th American Society of Retina Specialists Annual Meeting, October 8-12, 2021; San Antonio, Texas, USA.
- D Boyer et al., “Safety of intravitreal pegcetacoplan in geographic atrophy: results from the DERBY and OAKS trials.” Presented at the 39th American Society of Retina Specialists Annual Meeting, October 8-12, 2021; San Antonio, Texas, USA.
- AM Khanani et al., “The efficacy of avacincaptad pegol in geographic atrophy: GATHER1 and GATHER2 results.” Presented at the 55th Retina Society Annual Scientific Meeting, November 2-5, 2022; Pasadena, California, USA.
- GJ Jaffe et al., “C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial,” Ophthalmology, 128, 576 (2021). PMID: 32882310.
- QE Cheng et al., “Design Characteristics of Geographic Atrophy Treatment Trials: Systematic Review of Registered Trials in ClinicalTrials.gov,” Ophthalmol Retina, 2, 518 (2018). PMID: 31047604.
- K Csaky et al., “Report From the NEI/FDA Endpoints Workshop on Age-Related Macular Degeneration and Inherited Retinal Diseases,” Invest Ophthalmol Vis Sci, 58, 3456 (2017). PMID: 28702674.
- Ora, “Clinical Endpoints for Retinal Disorders” (2017). Available at: https://bit.ly/3rcRpk6.
- JM Colijn et al., “Enlargement of Geographic Atrophy from First Diagnosis to End of Life,” JAMA Ophthalmol, 139, 743 (2021). PMID: 34014262.
- TD Keenan et al., “Progression of Geographic Atrophy in Age-related Macular Degeneration: AREDS2 Report Number 16,” Ophthalmology, 125, 1913 (2018). PMID: 30060980.
- AS Lindblad et al., “Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26,” Arch Ophthalmol, 127, 1168 (2009). PMID: 19752426.
- SR Sadda et al., “Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration,” Retina, 36, 1806 (2016). PMID: 27652913.
- JS Sunness et al., “Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration,” Ophthalmology, 106, 1768 (1999). PMID: 10485549.
- JS Sunness, CA Applegate, “Long-term follow-up of fixation patterns in eyes with central scotomas from geographic atrophy that is associated with age-related macular degeneration,” Am J Ophthalmol, 140, 1085 (2005). PMID: 16376656.
- L Schmetterer et al., “Endpoints for clinical trials in ophthalmology,” Prog Retin Eye Res, [Online ahead of print] (2023). PMID: 36599784.
- RH Guymer, “Geographic Atrophy Trials: Turning the Ship around May Not Be That Easy,” Ophthalmol Retina, 2, 515 (2018). PMID: 31047603.
- M Abidi et al., “A Clinical and Preclinical Assessment of Clinical Trials for Dry Age-Related Macular Degeneration,” Ophthalmol Sci, 2, 100213 (2022). PMID: 36570624.
- RW Beck et al., “Visual acuity as an outcome measure in clinical trials of retinal diseases,” Ophthalmology, 114, 1804 (2007). PMID: 17908590.
- V Chong, “Endpoints: The Beginning of a New Treatment?” Ophthalmologica, 244, 365 (2021). PMID: 34348339.
- CJ Flaxel et al., “Age-Related Macular Degeneration Preferred Practice Pattern®,” Ophthalmology, 127, P1 (2020). PMID: 31757502.
- J Heier et al., “Efficacy of intravitreal pegcetacoplan in geographic atrophy: 24 month results from the phase 3 OAKS and DERBY trials.” Presented at the 55th Retina Society Annual Scientific Meeting, November 2-5, 2022; Pasadena, California, USA.
- DS Liao et al., “Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial,” Ophthalmology, 127, 186 (2020). PMID: 31474439.
- C Danzig et al., “Intravitreal avacincaptad pegol in geographic atrophy: post-hoc analysis of vision loss from the GATHER clinical program.” Presented at the 2023 Association for Research in Vision and Ophthalmology Meeting, April 23-27, 2023; New Orleans, Louisiana, USA.
- JS Heier et al., “Visual function decline resulting from geographic atrophy: results from the Chroma and Spectri phase 3 trials,” Ophthalmol Retina, 4, 673 (2020). PMID: 32199866.
- JS Sunness et al., “Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration,” Ophthalmology, 115, 1480 (2008). PMID: 18486216.
- LJ Wood et al., “Low luminance visual acuity as a clinical measure and clinical trial outcome measure: a scoping review,” Ophthalmic Physiol Opt, 41, 213 (2021). PMID: 33403668.
- Stealth Biotherapeutics, “ReCLAIM-2 Study Topline Results: A Phase 2 Study of Elamipretide Safety & Efficacy in Geographic Atrophy” (2022). Available at: https://bit.ly/487iOoe.
- AA Brunet et al., “Primary and secondary cone cell death mechanisms in inherited retinal diseases and potential treatment options,” Int J Mol Sci, 23, 726 (2022). PMID: 35054919.
- A Takahashi et al., “Photoreceptor damage and reduction of retinal sensitivity surrounding geographic atrophy in age-related macular degeneration,” Am J Ophthalmol, 168, 260 (2016). PMID: 27296489.
- AC Bird et al., “Geographic atrophy: a histopathological assessment,” JAMA Ophthalmol, 132, 338 (2014). PMID: 24626824.
- FG Holz et al., “Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from Classification of Atrophy consensus meetings.” Ophthalmology, 124, 464 (2017). PMID: 28109563.
- J Wang, GS Ying, “Growth rate of geographic atrophy secondary to age-related macular degeneration: a meta-analysis of natural history studies and implications for designing future trials.” Ophthalmic Res, 64, 205 (2021). PMID: 32721951.
- Business Wire, “Iveric Bio Receives FDA Agreement Under Special Protocol Assessment (SPA) for GATHER2 Phase 3 Clinical Trial of Zimura® in Geographic Atrophy Secondary to Age-Related Macular Degeneration” (2021). Available at: https://bit.ly/46ef4jg.
- AJMC, “Overview of GATHER1 and GATHER2 Studies: Part 1” (2023). Available at: https://bit.ly/3ZjgZk8.
- S Riedl et al., “The effect of pegcetacoplan treatment on photoreceptor maintenance in geographic atrophy monitored by artificial intelligence-based OCT analysis,” Ophthalmol Retina, 6, 1009 (2022). PMID: 35667569.
- M Pfau et al., “Progression of photoreceptor degeneration in geographic atrophy secondary to age-related macular degeneration,” JAMA Ophthalmol, 138, 1026 (2020). PMID: 32789526.
- RA Goldberg et al., “Assessment of Geographic Atrophy Lesion Progression in the Phase 3 DERBY and OAKS Trials.” Presented at the 40th American Society of Retina Specialists Annual Meeting; July 13-16, 2022; New York City, New York, USA.
- NGM Bio, “Topline Results from the CATALINA Phase 2 Trial of NGM621 in Patients with Geographic Atrophy Secondary to AMD” (2022). Available at: https://bit.ly/3EGPDuG.
- SR Sadda et al., “Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of Atrophy report 3,” Ophthalmology, 125, 537 (2018). PMID: 29103793.
- SB Velaga et al., “Correlation between fundus autofluorescence and en face OCT measurements of geographic atrophy,” Ophthalmol Retina, 6, 676 (2022). PMID: 35338026.
- JP Ehlers, “Exploration of machine learning–enhanced compartmental retinal integrity assessment for progression risk and treatment response in the GATHER1 study.” Presented at the 45th Macula Society Annual Meeting; June 8-11, 2022; Berlin, Germany.
- MG Nittala et al., “Association of pegcetacoplan with progression of incomplete retinal pigment epithelium and outer retinal atrophy in age-related macular degeneration: a post hoc analysis of the FILLY randomized clinical trial,” JAMA Ophthalmol, 140, 243 (2022). PMID: 35113137.
- A Lowenstein et al., “Geographic atrophy progression following treatment with avacincaptad pegol from the GATHER1 study.” Presented at the 3rd Retina World Congress; May 12-15, 2022; Fort Lauderdale, Florida, USA.
- SH Künzel et al., “Determinants of quality of life in geographic atrophy secondary to age-related macular degeneration,” Invest Ophthalmol Vis Sci, 61, 63 (2020). PMID: 32462198.
- F Hou et al., “Evaluating the performance of the quick CSF method in detecting contrast sensitivity function changes,” J Vis, 16, 18 (2016). PMID: 27120074.
- F Vingopoulos et al., “Measuring the contrast sensitivity function in non-neovascular and neovascular age-related macular degeneration: the quantitative contrast sensitivity function test,” J Clin Med, 10, 2768 (2021). PMID: 34202569.
- GR Jackson et al., “Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration,” Invest Ophthalmol Vis Sci, 55, 1427 (2014). PMID: 24550363.
- M Kimel et al., “Functional Reading Independence (FRI) Index: a new patient-reported outcome measure for patients with geographic atrophy,” Invest Ophthalmol Vis Sci, 57, 6298 (2016). PMID: 27893095.
