The biggest challenge in combatting corneal disease is repairing the stroma. It makes up nine-tenths of the corneal thickness and imparts most of the strength, shape and even the transparency of the cornea. Dystrophies, ectactic disorders and even scars disrupt the stromal anatomy and physiology, destroying its transparency and leading to vision loss. Being able to repair it would be a game-changer but, until now, the only option has been to replace it through corneal transplantation. Efforts have been made to recreate the stroma in vitro, but they’ve all met with failure (1). The stroma may be mostly collagen, but its complex ultrastructure has meant that recreating it, or finding a suitably strong and transparent replacement, has been impossible to date. An alternative approach – recreating the stroma with stem cells – has shown far more promise.
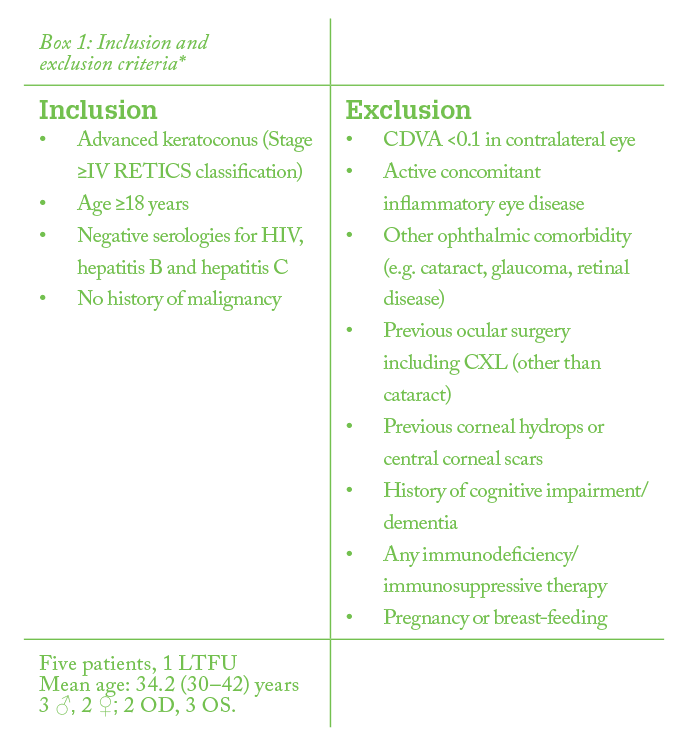
Corneal stromal stem cells (CSSCs) do exist, and at face value, they’re a great option for treating corneal dystrophies, scars and ectasias. They’re already corneal cells, with a more directed differentiation profile (2). However, there are three problems with CSSCs: i) few are present in any given cornea, ii) they’re more technically challenging to harvest (and doing so damages the cornea), and iii) they’re more difficult to cultivate and expand in cell culture – all of which precludes their autologous use. Compare this with extraocular mesenchymal stem cells (MSCs). MSCs (from both ocular and non-ocular sources) have already shown great promise: in rabbits, human MSCs can survive, differentiate into adult human keratocytes and produce new human collagen within the rabbit stroma, without an inflammatory reaction (2)(3)(4)(5)(6). Further, mouse MSCs (in mice) have been shown to improve corneal scars by stroma remodeling, and to improve corneal transparency in animal models of corneal dystrophies and metabolopathies through collagen reorganization and catabolization of accumulated proteins, respectively (7)(8)(9)(10)(11)(12). In terms of potential clinical application, human adipose-derived adult stem cells (ADASCs) could be the MSC of choice; not only can they differentiate into multiple stem cell types, but also the source material, adipose tissue, is easily obtained by liposuction – and ADASCs can be retrieved with high efficiency (2). In other words, a patient can undergo liposuction, have their own ADASCs harvested, and have those stem cells applied to the cornea – in theory. The big questions are: can it work? And is it safe? My colleagues and I decided to perform a small, phase I safety and tolerability clinical trial in five patients with keratoconus (see Box 1: Inclusion criteria) as a first step to finding out (13).
The process of ADASC cell harvesting is described in Figure 1 and the microscopic appearance of these cells is depicted in Figure 2. Once we had prepared the stem cells – three million in 1 mL of saline – we used a 60-kHz IntraLase iFS femtosecond laser to create a 9.5 mm diameter, half-depth (of an OCT-determined thinnest pachymetry point) intrastromal pocket, which ended with a 30° anterior side-cut as a corneal incision. Next, we opened the pocket by blunt dissection with a lamellar dissector, and a 1-mm corneal paracentesis was made to reduce IOP and increase the volume of cells that could be introduced into the corneal pocket. ADASCs suspended in 1 mL saline were injected into the pocket through a 25-G cannula. No corneal suturing was required in any cases, and the surgery was completed with the administration of topical steroids and antibiotics (Tobradex, Alcon). The postoperative care and assessment schedule is summarized in Figure 3. So what did we find? Of the five patients enrolled, one was lost to follow-up after one month and was excluded from our analyses. No complications occurred during surgery – or throughout the six-month follow-up (the patient that dropped out had experienced no complications by one month, and later indicated no subjective negative findings or outcomes when contacted directly about this), and full corneal transparency was achieved within 24 hours in all cases (Figure 4). All patients’ visual function improved: both uncorrected visual acuity (UVA) and corrected distance visual acuity (CDVA) improved by an average of one line, and by two lines of distance rigid contact lens visual acuity (CLVA) – although this should, in theory, be attributed to the surgical procedure and not the stem cells as the improvements were rapid – and the stem cells had not differentiated into adult keratocytes at this early point. Manifest and topographic keratometry remained stable, and anterior-segment OCT showed not only new collagen production in the stromal pocket (Figure 5), but also a mean improvement in corneal thickness of 16.56 µm. One important question to answer was if the implanted stem cells survived – is their effect likely to be in the short term, or over a more prolonged period? Encouragingly, confocal biomicroscopy (Figure 6) performed at three months confirmed that they survived at the surgical plane, and by six months these cells had adopted a fusiform shape and did not appear different to cells in other stromal planes. Finally, IOP and endothelial cell density remained stable. So what does this mean for the future treatment of corneal disorders? One has to bear in mind that this was a small, uncontrolled, unmasked pilot study with only a six-month follow up-period – and that any future studies would need to improve on these aspects. But despite these provisos, the clinical corneal stromal implantation of autologous ADASCs appears to: i) be non-immunogenic and without adverse event, ii) result in the production of a (low) amount of new collagen. And the cells appear to survive in vivo. The procedure might provide an alternative to corneal transplantation in patients who have been diagnosed with keratoconus that is too advanced to treat with corneal collagen cross-linking (and might also avoid many of the risks inherent with this form of surgery, principally graft rejection). It would also be interesting to speculate what impact intrastromal autologous ADASC transplantation might have on the natural course of mild or moderate keratoconus. A new door has opened for the clinical treatment of corneal disorders – the next few years should be exciting times. Jorge Alió is Professor and Chair of Ophthalmology, Miguel Hernández University of Alicante, and founder of VISSUM Corporation. Jorge Alió del Barrio is a cornea, cataract and refractive surgeon at VISSUM Corporation, and an honorary Professor at Miguel Hernández University.
References
- JW Ruberti, JD Zieske, “Prelude to corneal tissue engineering—gaining control of collagen organization”, Prog Retin Eye Res, 27, 549–577 (2008). PMID: 18775789. MP De Miguel et al., “Frontiers in regenerative medicine for cornea and ocular surface”, in: Rahman A, Anjum S, eds. Frontiers in Stem Cell and Regenerative Medicine Research. 1st ed. Vol 1. Cambridge, PA: Bentham e-Books; 92–138 (2015). F Arnalich-Montiel et al., “Adipose-derived stem cells are a source for cell therapy of the corneal stroma”, Stem Cells, 26, 570–579 (2008). PMID: 18065394. JL Alió del Barrio et al., “Biointegration of corneal macroporous membranes based on poly(ethyl acrylate) copolymers in an experimental animal model”, J Biomed Mater Res A, 103, 1106–1118 (2015). PMID: 24910285. JL Alió del Barrio et al. “Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model”, Exp Eye Res, 132, 91–100 (2015). PMID: 25625506. L Espandar et al., “Adipose-derived stem cells on hyaluronic acid-derived scaffold: a new horizon in bioengineered cornea”, Arch Ophthalmol, 130, 202–208 (2013). PMID: 22332213. SK Mittal et al., “Restoration of corneal transparency by mesenchymal stem cells”, Stem Cell Reports, 7, 583–590 (2016). PMID: 27693426. B Demirayak et al., “Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury”, Exp Eye Res, 151, 227–235 (2015). PMID: 27567556. Y Du et al., “Stem cell therapy restores transparency to defective murine corneas”, Stem Cells, 27, 1635–1642 (2009). PMID: 19544455. H Liu et al., “Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice”, PLoS One, 5, e10707 (2010). PMID: 20502663. VJ Coulson-Thomas et al., “Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice”, Stem Cells, 31, 2116–2126 (2013). PMID: 23897660. WW Kao, VJ Coulson-Thomas, “Cell therapy of corneal diseases”, Cornea, 1, S9–S19 (2016). PMID: 27631350. JL Alió del barrio et al., “Cellular Therapy With Human Autologous Adipose-Derived Adult Stem Cells for Advanced Keratoconus”, Cornea, 36, 952–960 (2017). PMID: 28486314.
