When it comes to newly approved treatments, real-world evidence is always eagerly anticipated: how safe and effective is it really, outside the confines of the highly-controlled world of clinical trials? Iluvien (fluocinolone acetonide intravitreal implant) has been available in the European market since 2013 “for the treatment of vision impairment associated with the chronic diabetic macular edema (DME), considered insufficiently responsive to available therapies” – and an interim analysis of the Iluvien Registry Safety Study (IRISS), has recently been published (1). Iluvien elutes therapeutic drug concentrations for up to three years – and poor patient regimen compliance in the real world is something that often stops outcomes in clinical practice matching those in clinical trials. So can the IRISS results match those of the chronic DME population in its Phase III trial, FAME?
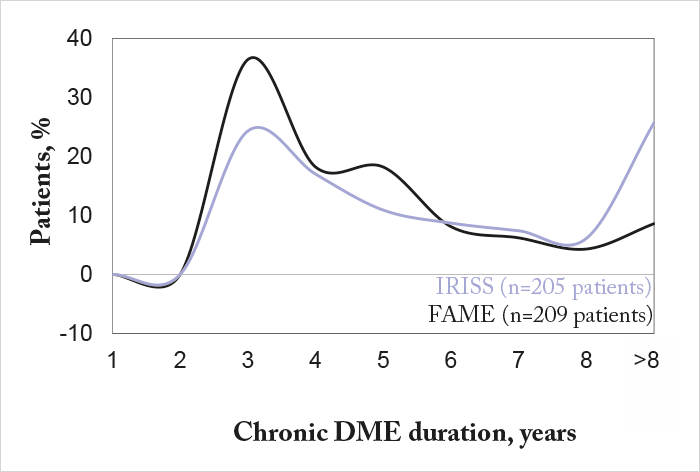
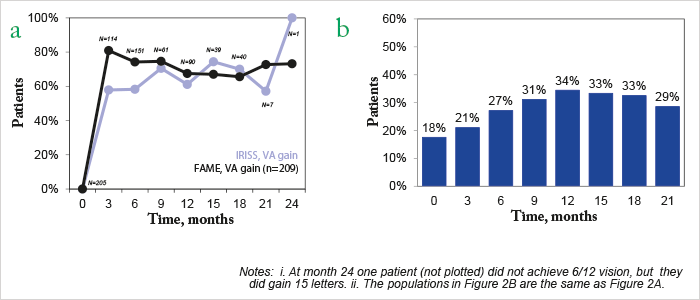
Some context. IRISS is an ongoing, open-label, five-year registry study of Iluvien that involves 37 sites in Europe and 800 patients with chronic DME. At its most recent interim analysis in April 2016, 328 eyes from 292 patients had been followed up for a mean duration of 281.9 days (range 3–763), and consistent with the FAME study results (2), around 60 percent of patients experienced visual acuity (VA) improvements after six and 12 months (1). So far, so good? It’s important to compare the patient demographics in IRISS and FAME – 25.7 percent of patients in IRISS had chronic DME for >8 years compared with 8.6 percent of FAME patients (Figure 1). Patients in IRISS received Iluvien late: 98.8 percent had received prior DME treatment (75.9 percent prior laser treatment (focal, grid, or PRP); at least two-thirds had received prior intravitreal anti-VEGF injections), demonstrating that Iluvien was being used as a last-line therapy. Three months after implantation, 58 percent of patients experienced gains in VA, and 21 percent had achieved 6/12 vision – the minimum legal requirement for driving in the EU (Figure 2).
But as in FAME, Iluvien hasn’t escaped the usual caveats associated with steroid therapy: IOP elevation and cataract formation. At interim analysis, 18.4 percent of patients in IRISS required IOP-lowering therapy, with 0.6 percent needing filtration surgery. IOP increased ≥10 mmHg in 9.9 percent of patients, and by 30 mmHg or more in 8.2 percent. Of the 51 phakic eyes in IRISS, five (9.8 percent) developed treatment-emergent cataract and 10 (19.6 percent) required cataract extraction. The current data from IRISS provides insight in Iluvien’s safety and efficacy in the real world in patients with chronic DME – and it appears to be broadly similar to what it achieved with “chronic patients” in the clinical trial setting. With the next data extraction planned for 2017, it will be interesting to see how things play out…
References
- S Taylor et al., “Changes in intraocular pressure after Iluvien (190 micrograms fluocinolone acetonide) – real-world experiences following usage in three European countries”. Poster presented at the Royal College of Ophthalmologists Annual Congress; May 24–26, 2016; Birmingham, England. Poster #110. PA Campochiaro et al., “Long-term benefit of sustained delivery fluocinolone acetonide vitreous inserts for diabetic macular edema”, Ophthalmol, 118, 626–635 (2011). PMID: 21459216.
