Diabetic maculopathy is the most common cause of visual impairment in individuals with diabetes. If a diabetic macular edema (DME) is diagnosed, the ophthalmologist needs to explain what maculopathy is, why it develops and which therapy modalities are available to the patient. Such counseling may improve compliance with clinic visits and treatment. The current standard of care for the treatment of DME is the intravitreal injection of anti-VEGF-inhibitors (ranibizumab and off-label bevacizumab). Studies have shown a significant advantage over focal laser photocoagulation, although laser therapy continues to have a role for patients without center involvement DME and good visual acuity. To maintain a therapeutic effect, multiple anti-VEGF injections are required. The guidelines recommend monthly anti-VEGF injections given for at least three consecutive months, followed by monthly assessment visits including visual acuity, fundoscopy and spectral-domain optical coherence tomography (SD-OCT), at least in the first year. Re-treatments are required until the macular is dry or until there is no further improvement. The treatment and follow-up regimen is considered a burden to the patient, which makes the consideration of alternative therapeutic modes attractive.
Intravitreal steroid injections are an alternative way for treating patients with DME. The long-acting steroid implant fluocinolone acetonide (Iluvien) is of special interest because of the reduced frequency of treatment required in comparison to anti-VEGF therapy, which may indicate a significant benefit.
Iluvien is currently approved in several European countries for the treatment of chronic DME in patients who do not demonstrate a sufficient response to available therapies.
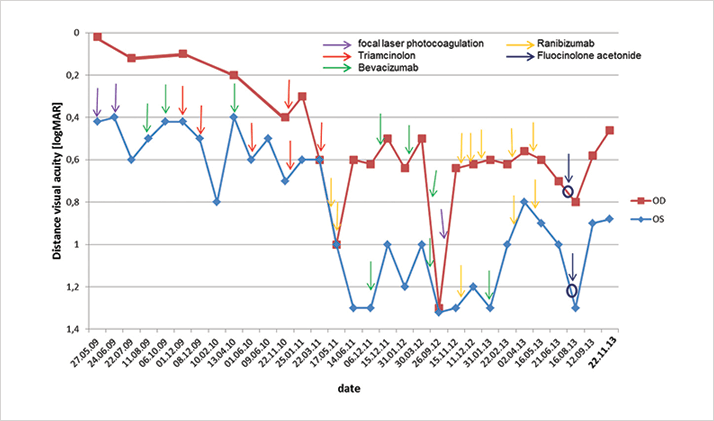
Case presentation Here we discuss our experience with an 87-year-old female patient presenting with diabetic retinopathy. Management and outcome From her initial consultation in 2009 through to 2013, the patient was treated with focal laser photocoagulation and multiple intravitreal steroid (triamcinolone) and anti-VEGF (bevacizumab and ranibizumab) injections in both eyes. Figure 1 outlines the treatment regimen administered between 2009 and 2013 and shows an insufficient response.
As a result of persistent DME, Iluvien was implanted in both eyes in August 2013. Follow-up examinations included visual acuity, OCT and IOP measurements. Figure 2 shows OCT images three months post-implantation, demonstrating improvements in visual acuity (OD = 0.7 logMAR to 0.46 logMAR; OS = 1.0 logMAR to 0.88 logMAR) and a decrease in central retina thickness (OD = 272 µm to 257 µm; OS = 312 µm to 263 µm). Meanwhile, IOP remained stable in both eyes compared with preoperative measurements (at around 12-13 mmHg).
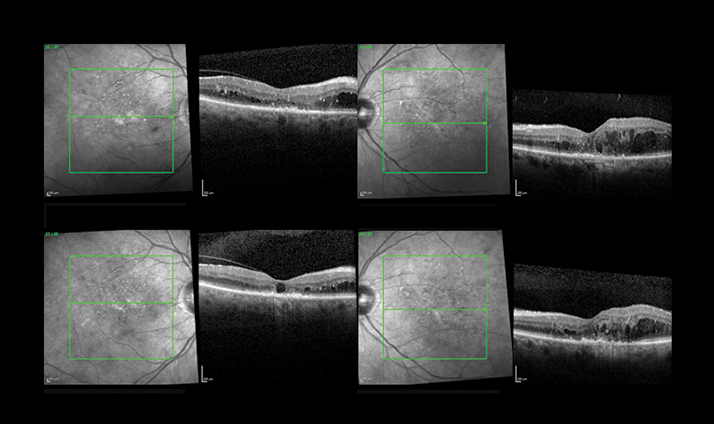
Discussion DME is characterized by retinal thickening in the macula observed by fundoscopy and OCT. The condition can be debilitating, in particular if inadequately treated. The introduction of intravitreal pharmacotherapy regimes has been a significant advance in the treatment of DME. However, regular follow-up examinations and re-treatments are required for an effective response to treatment, making alternative treatment options attractive. One intravitreal implant containing 190 µg of fluocinolone acetonide (Iluvien), provides sustained treatment over approximately three years, obviating the need for repeat procedures during the treatment window. The long treatment effect also extends the frequency of follow-up examinations and re-treatments. We believe that this sustained release implant offers a number of benefits to DME patients when compared with other available intravitreal treatments, including reduced treatment and follow-up frequency; lower cumulative endophthalmitis risk owing to lower injection frequency, and reduced anxiety amongst patients fearful of ocular injections. Overall, we believe that treatment with Iluvien improves patient quality of life. In this case, we have also demonstrated improvement in functional results in a patient with therapy-resistant DME.
