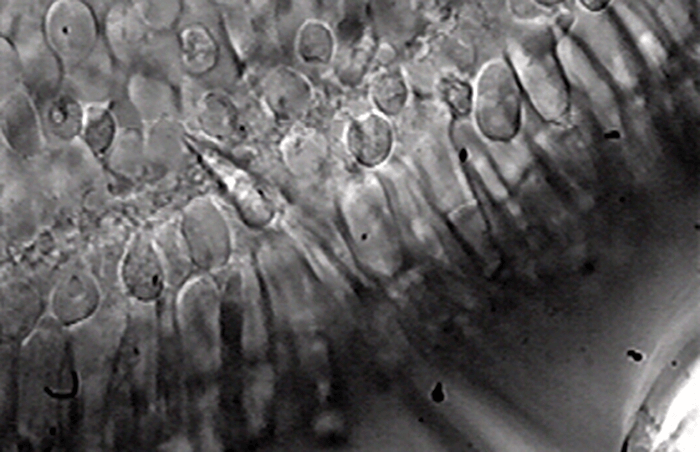
- Foveal signaling and neurophysiological function has long been poorly understood, partly because of long-standing technical challenges; patch clamp electrophysiology and transient gene expression techniques have now permitted the first detailed investigation of how the fovea functions at a cellular and circuit level
- Unexpected findings include the observation that perceptual differences in temporal sensitivity between foveal and peripheral vision originate in the first stage of visual processing i.e. phototransduction in the cone photoreceptors
- Another striking finding is that the responses of the dominant output neurons in the fovea are minimally modulated by synaptic inhibition, unlike most neural circuits
- The study has caused a re-evaluation of foveal function at the cellular and neuronal circuit level and may inform future therapeutic strategies for visual disorders
Relative to the rest of the retina, the fovea exhibits striking anatomical and structural specializations that not only support spatial and chromatic sensitivity, but also enable maximal visual acuity. So although the fovea subtends only a tiny part of the visual field – about the size of a thumbnail at arm’s length – it accounts for approximately half of the information sent to the visual cortex. Given this striking anatomical specialization, should we also expect to find differences between foveal and non-foveal neural cells at the physiological level?
Certainly, the temporal sensitivity – the ability to distinguish changes in visual inputs over time – of foveal circuits is known to be relatively low compared with that of peripheral retinal cells. In fact, foveal temporal sensitivity determines key features of the modern environment, such as celluloid film frame rates and computer monitor refresh rates. Aside from this, we know very little about neural signaling in the fovea; most of our knowledge stems from sparse, decades-old literature. The dearth of data is mainly due to the historical challenges associated with foveal research, not least the technical difficulty of recording intracellular electrical activity in foveal neurons. Moreover, animal models are scarce and expensive, as the fovea is found only in diurnal primates. Only a few labs in the world have worked in this field, and our understanding of neural function in this specialized retinal region remains limited.
New approaches
To me, the sparse literature on foveal function seemed like an incredible opportunity to explore relatively uncharted territory in visual signaling. I had no background in retinal research – but that was something of an advantage, as I wasn’t fully aware of the technical challenges that lay ahead! So I joined Fred Rieke’s lab at the University of Washington (UW), and began generating patch-clamp recordings from neurons in the fovea. This technique (Figure 1) allowed me to measure light-induced electrical responses from single output neurons called ganglion cells. I could measure both the output of the foveal neurons and also the inputs they received from upstream neurons, and compare these measurements with those recorded from their counterparts in the peripheral retina. To complement the electrophysiological results with detailed anatomical investigation, we used gene expression techniques, such as particle-mediated gene transfer – not previously used in primate fovea – in collaboration with Mrinalini Hoon from Rachel Wong’s laboratory at UW, This allowed transient expression of proteins in various ganglion cells in the fovea.In essence, my aim was to understand the physiological basis of the differences between foveal and peripheral vision at the level of perception. It wasn’t straightforward but it was exciting. Technical challenges included the development of in vitro approaches that minimized foveal damage while permitting reliable measurement of light-evoked responses in the foveal photoreceptors and in the dominant class of output neurons (midget ganglion cells). We were able to measure responses to a visual stimulus from midget ganglion cells within the range of contrast sensitivities previously reported in in vivo recordings. This enabled us to make direct comparisons of the physiological properties of foveal and peripheral retinal neurons. We reported the first intracellular recordings of light-evoked responses from photoreceptors and ganglion cells in the fovea and the first structure-function correlation in the fovea. Patch-clamp electrophysiological methods were crucial for directly measuring excitatory and inhibitory synaptic inputs to foveal ganglion cells and comparing those with ganglion cell outputs (action potentials). When we commenced this research, the published literature showed a complete lack of intracellular recordings from foveal neurons, so it really was uncharted territory – we didn’t know what we would find.
New findings
In brief, our work provided the first glimpse into the cellular, synaptic and circuit mechanisms of foveal function, and it turns out to be very different from the operation of non-foveal retina (1). Firstly, we found that foveal midget ganglion cells expressed fewer inhibitory postsynaptic receptors on their dendrites than their peripheral counterparts. This meant that responses of midget ganglion cells in the fovea are only minimally shaped by synaptic inhibition – very surprising, because integration of excitatory and inhibitory signals is a key feature of most neural circuits in the brain, including peripheral midget ganglion cells. Indeed, a major research theme has been the extent to which the computational specialization of non-foveal retinal circuits relies on signals from inhibitory retinal neurons (2). For us to show that the responses of foveal ganglion cells are not significantly modulated by either pre- or post- synaptic inhibition, therefore, was most unexpected. Effectively, these cells participate in a neural circuit that operates independently of synaptic inhibition, which is extremely unusual.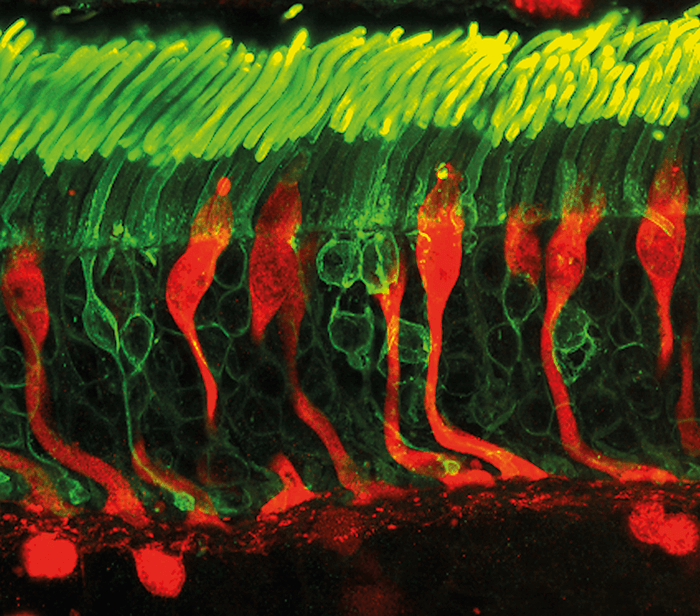
Secondly, and contrary to our expectations, we showed that the different temporal sensitivities of the fovea and peripheral retina did not arise from differences in synaptic inhibition. Rather, they originated in the cone photoreceptors. Foveal cones exhibit response profiles two-fold slower than those in peripheral retina, which is nearly identical to the difference in perceptual sensitivity observed between the foveal and peripheral vision. Thus, lower levels of synaptic inhibition do not seem to be behind the lower temporal sensitivity of the fovea. Instead, it seems that the “frame-rate” capacity of our visual system is set by the very first neurons in the visual pathway. So, to recap, there were several surprises in this study. We revealed a key difference between foveal and peripheral midget ganglion cells in terms of synaptic inhibition; the minimal role of synaptic inhibition in the foveal midget ganglion cells was completely unforeseen, given the importance of balanced excitation and inhibition for the operation of most other neural circuits in the brain. Additionally, we found that differences in temporal sensitivity between the two retinal regions may originate in the cones within the phototransduction cascade. The differences provide a simple explanation for the observation that foveal vision is less sensitive to rapidly varying light inputs than peripheral vision, and has significantly changed our understanding of foveal function. Previous models of visual function assumed that differences between foveal and peripheral vision originated in the neural circuits that received input from cone photoreceptors. The heterogeneity of cone responses that we observed means that we must re-evaluate old models – and the conclusions drawn from them. Our work clearly illustrates that the fovea is not only structurally specialized, but also employs different computational strategies compared to peripheral retina. This distinction is also important when considering how visual computations are partitioned between the retina and higher visual centers. In sum, our findings have revised our understanding of how the fovea operates at a cellular and circuit level.
New horizons
Our study offers the first glimpse into how the fovea works at a cellular and circuit level and has opened up a whole new research field. The novel application of gene transfer approaches that we’ve described may permit a wide range of transient genetic manipulations that will allow us to understand the properties of other cell types in the fovea. And that will enable us to fill the void in our knowledge of this crucial component of the human eye. Indeed, given the distinct functional properties of foveal neurons we have revealed, the lack of significant functional data on foveal circuits, and the implications for the division of computational labor between retina and cortex, our work should be of broad interest to scientists studying visual signal processing. I am certainly pursuing this unique opportunity to unravel the mechanistic basis of other aspects of foveal vision! The data we present also have implications beyond the theoretical. For conditions involving central vision loss, current therapeutic strategies – including the design of visual prosthetics – are based on studies in peripheral retina, or in retinas from model systems that lack a fovea. The underlying assumption has been that the fovea is a scaled-down version of the peripheral retina, but our study shows that the computational architecture and visual processing mechanisms of the fovea are dramatically different from non-foveal retina. Our new understanding may assist strategies to alleviate important visual deficits, such as macular degeneration. After a Physiology degree at the University of Calcutta, Raunak Sinha worked at the Tata Institute of Fundamental Research in Mumbai before undertaking his PhD at the International Max Planck Research School in Göttingen, in the Department of Membrane Biophysics at the Max-Planck Institute for Biophysical Chemistry headed by Nobel Laureate Erwin Neher. His doctoral thesis was awarded the Otto Hahn medal. Raunak works in Fred Rieke’s laboratory at the Howard Hughes Institute, Seattle, WA.References
- R Sinha et al., “Cellular and circuit mechanisms shaping the perceptual properties of the primate fovea”, Cell, 168, 413-426. PMID: 28129540. RH Masland. "Vision: Two speeds in the retina", Curr Biol, 27, R303–305 (2017). PMID: 28441563.2
