The Czech writer Karel Čapek is recognized as being the first person to coin the term “robot,” in his play R.U.R. (Rossum’s Universal Robots), first published in 1920. The etymology of the word is worth exploring, stemming from the Czech word robota, meaning drudgery. If we go forward in time by just 30 years, we see Isaac Asimov capturing the public’s imagination with science fiction novels like I, Robot, and that decade, the 1950s, saw many ambitious predictions of what the distant future – the year 2000 – would bring in terms of robot service to humankind.
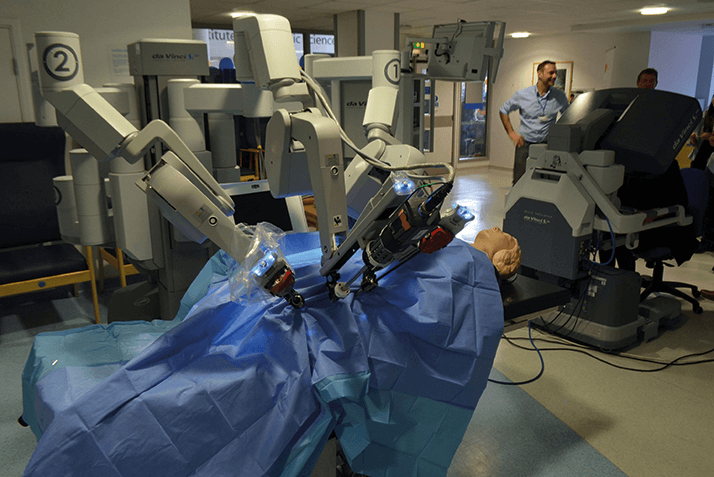
Ultimately, the timescales imagined in the 1950s were a little too ambitious, although the field of medicine did see its first robot in 1984: Arthrobot. Arthrobot was used to manipulate patients’ knee joints into the appropriate position for each section of knee arthroplasty work. The alternative was for a human assistant to hold the limb in exactly the right place for extended periods of time – something that requires precision, rapidly becomes tiring, and is certainly boring: drudgery. If we fast-forward to the year 2000, domestic robots were still nowhere to be seen – it was another two years until the first iRobot Roomba vacuum cleaner launched. Though the sci-fi predictions 50 years prior fell short when it came to household chores, they were closer to the mark when it came to surgery. By 2000, a number of robots capable of performing multiple surgical procedures were available, one of which I’ve actually managed to evaluate (in vitro) in ophthalmology: the da Vinci robotic surgical system (Figure 1).
Better than the best of humans?
Robotic surgery within ophthalmology holds a lot of promise (Table 1). Yes, there’s a general trend towards increasing automation, but I think robotic surgery will come into our field in the future for different reasons.Precision One of those reasons will be because this type of technology has high precision and stability, and can filter out tremors, which should allow ophthalmic surgeons to carry out procedures that are beyond what we can do right now. Robotic assistance also means that you can scale both movements and speed, enabling you to perform some extremely delicate tasks (1). In general, if you think about surgery, we surgeons are good when the tissue is fairly healthy. When the tissue is very friable – even though it may need to be preserved – it becomes much more difficult to operate. Take the example of the retina that’s partially degenerated. A general approach is to remove that part and try to deal with the healthy tissue – but there are cases of retinal dystrophy where even that wouldn’t necessarily be possible. If you could safely deal with that friable tissue, using a high-precision instrument that filters most of the movement and potential damage that we introduce with tremor, then you could open up the possibility of treating situations that are currently very difficult to approach.
Repetition, and the lost art of suturing But of course, robots were originally conceived as devices that could automate repetitive, boring tasks – drudgery – and today’s surgical robots are particularly good at that. So by definition, any operation that has been honed down to its very basics can be automated, potentially speeding patient turnover and reducing the requirement for space. One could think about certain parts of cataract surgery, which today use femtosecond lasers. The procedures that the femtosecond laser isn’t particularly good at doing – the corneal incision – but also the capsulorhexis, could be taken over by a robotic system. Another “lost art” in the current generation of ophthalmic surgeons: suturing. It’s not really a characteristic of most ophthalmic procedures, but there are still occasionally situations that require it, and this is where a robot could take over the repetitive motions involved and place sutures at the ideal depth and with the required strength.
Education Then there’s the bigger picture: the education of surgeons and the dissemination of new techniques. There is now a general trend to first train physicians on simulators. If the simulators – and the robots – have all the different steps necessary for the procedure pre-programmed into them, then it should be relatively simple for a surgeon to transition from one to the other. But this brings an even bigger opportunity; the ability to record new procedures developed on the robot (Figure 2) and the rapid dissemination of that information to a multitude of centers. I can envisage those centers using (at least initially) the procedure that was pre-programmed, then probably adapting it to each surgeon’s own requirements. I think, as new approaches develop, using the robotic approach could allow the adoption of new surgical approaches far more quickly than is possible today.
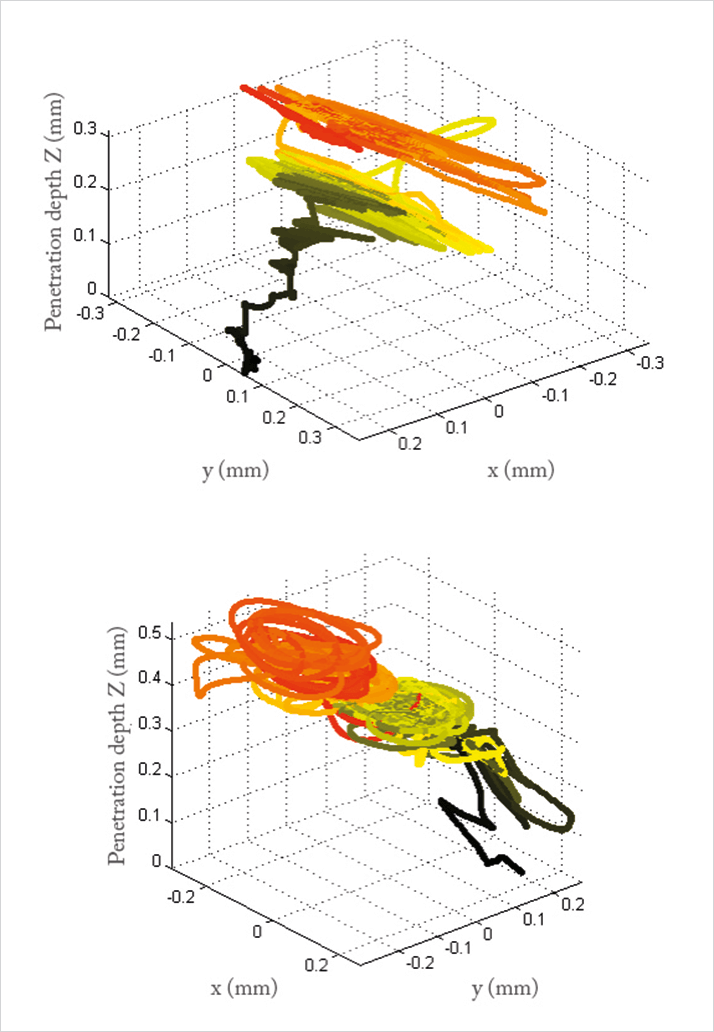
The challenges Robotic assistance is not without issues that need to be carefully considered in order to be resolved (Table 1). There are a number of inherent workflow needs to consider. For example, the robot needs to have its functionalities selected and adapted for each of its required tasks, in sequence. Furthermore, the sequence identity needs to be determined using non-visual cues (although intraoperative imaging may help with that), so it has to be carefully planned and programmed. There are a number of surmountable issues that need to be dealt with (Table 1), but ultimately, robotics devices need to be inherently intuitive, with a minimal learning curve, to maximize their appeal to ophthalmic surgeons. The surgeon is looking down a microscope, or at a screen. So as we’re devising a task to be carried out with robot assistance, it has to make sense. The robot must carry out a sequence of steps that are logical for the surgeon as he or she is assisted in performing the surgery, or monitors what is going on in the eye. Anything that deviates from this logical progression (or indeed any major shift in the surgical focus) should be announced by non-visual cues.
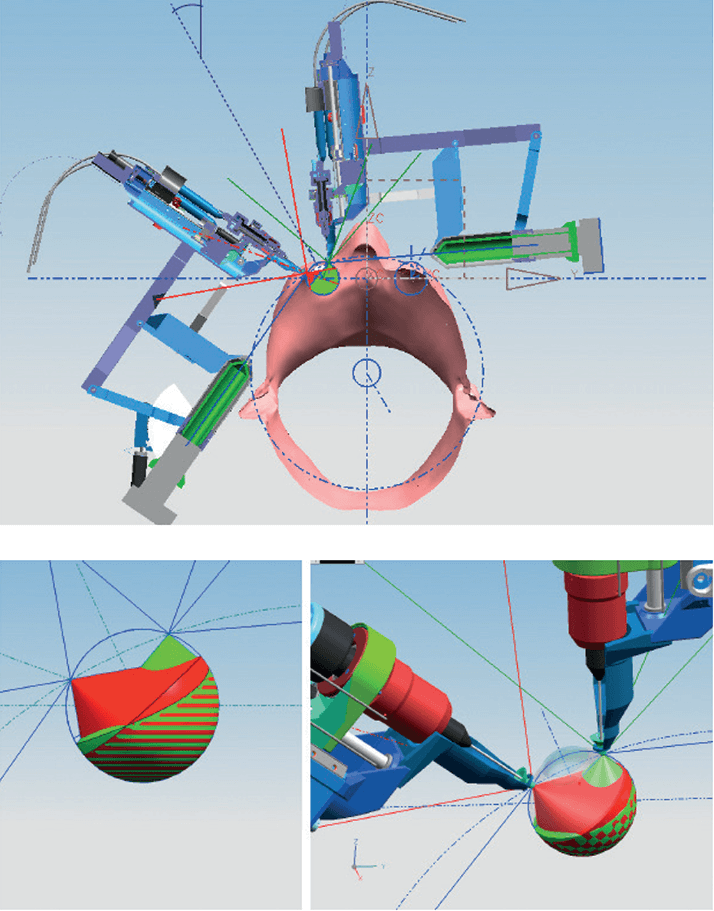
A robotic system suitable for eye surgeons Da Vinci is an impressive multipurpose robotic surgical device. The camera on it gives you sufficient viewing of the eye to enable you to perform corneal surgery and place sutures. But it’s a very large machine with a big arch. Why? Because it’s designed for abdominal surgery, where the robot needs not only to wrap around people’s bodies, but also to be anchored to the floor of the operating room. This helps its frame to remain rigid and stable – giving the robot arms a fixed reference in order to determine the position of its instruments. But this is not the right approach for ocular surgery. The frame of reference is too limited; you need an apparatus that wraps around the head, so that you can approach the eye and have precision. So that’s the approach that we’ve been taking in Eindhoven with Preceyes: indirect devices that, in essence, work as a robotics system (Figure 3, and gallery below).
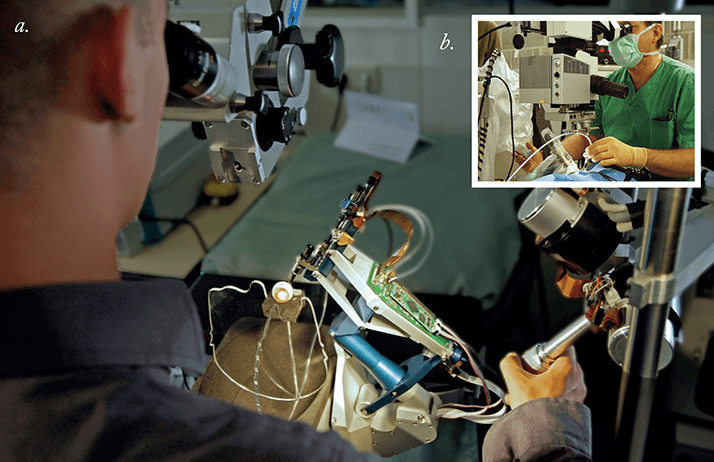
What we decided to do was develop a robotic assistive device that could accommodate the heads of 98 percent of Europeans (in terms of the size of the head, the position of the eyes, and so on) and help facilitate certain tasks during surgery. We saw this as the simplest way to introduce robotic devices into the surgical world. We’ve created a system that can be fitted to virtually any surgical table and positioned to suit to any size or type of head, so that you can place your instrument in the right location and have a very broad intraocular reach (Figure 4).
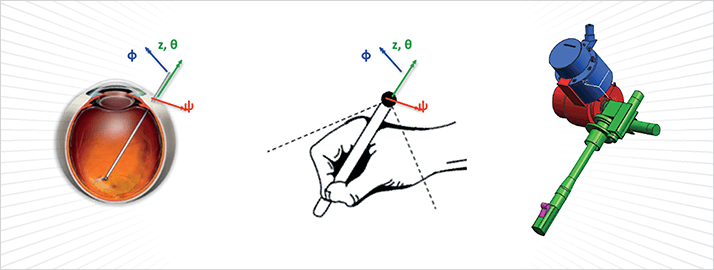
Easing the strain on surgeons Preceyes was initially developed for vitreoretinal surgery, but could be adapted for cataract surgery in the future. The controller is simple to operate – just like using a pen – and has a precision of under ten microns, ten times better than humans can achieve (Figure 5). It can filter out tremor and scale motions – faster when far from the retina, slower when nearing critical surgical planes for greater precision. It also has positional memory, which means you can leave the instrument and have a breather! This is not trivial at all – the biomechanical and physiological factors involved in ophthalmic surgery mean that, after 15 years of surgery, an average of 70 percent of microscopists in ophthalmology have shoulder pain, half have neck pain, half have lower back pain, and 40 percent have upper back pain (2–4). Being able to rest will make a big difference!
Our current research Retinal surgery is a complex set of steps, because it’s hard to standardize all of the different procedures – so we have two aims. The first is to look for new applications that can make use of our robotic system’s enhanced capabilities – like the cannulation of retinal veins to directly administer treatment. For example, in cases of retinal vein occlusion (RVO), tissue plasminogen activator (tPA) or other lytic agents can be administered to the cannulated vein to lyse the clot in situ, rapidly resolving the RVO with a minimal dose of the drug. A video demonstration of the cannulation of a small vein (in the chorioallantoic membrane of a fertilized chicken egg) is illustrated in Video 1. Another possible application is the delivery of cells into the subretinal space for cell therapy – either through the vitreous or via a transscleral approach (Video 2), and we will be exploring the device for transfer of viral vectors too.
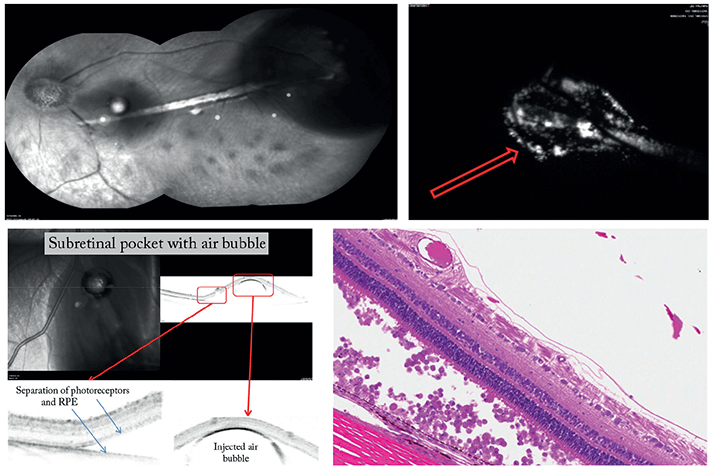
We’re trying to drill down each individual step in vitreoretinal procedures, so we can automate some of them – such as a retinal peel, pan-retinal laser, air-fluid exchange, and even core vitrectomy. Routine parts of surgery can be carried out within safe and appropriate parameters thanks to the precision of the robotic device, meaning that the surgeon’s attention is directed to more critical aspects of the surgery. So while a core vitrectomy is carried out, the surgeon can make last-minute adjustments to the surgical plan, supervise the setup of critical equipment, or verify the dose of a drug or cell therapy for intraocular delivery. This can reduce the length of operations, increase efficiency, and maximize turnover. Automation can also help deliver successful outcomes of difficult surgical steps. For example, we’ve already shown that the successful creation and catheterization of a transscleral subretinal bleb can be enhanced using our device (Figure 6). In an experimental model, experienced medical doctors (MDs), inexperienced MDs and non-MDs were all able to carry out the procedure, and after some training, non-MDs managed to achieve success rates similar to those of experienced surgeons. This suggests that robotic-assisted surgery can quickly transfer the knowledge needed to perform tricky procedures, allowing inexperienced surgeons to operate safely.
We’re also looking at the interface of robotics with visualization systems. There’s a lot of talk of intraoperative OCT (iOCT), and I think that’s a match made in heaven. Most surgeons use the iOCT to judge the quality and completeness of their surgery – for example, to see if they need to do some more retinal peeling. Preceyes gives you the ability to do that in real-time, so as you’re doing the surgery, you can follow it with OCT. We’re only starting to look at that at the moment, but it holds a lot of potential.
Why cost is a consideration – and how this might change the practice of ophthalmic surgery There’s always the issue of cost. Even if everything’s rosy and fantastic with robots in the future, that won’t get the machines sold. The adoption of equipment, depends (in part) on the ability to prove to hospital managers that it will save them money. So in that regard, robotics allows miniaturization – you need less space around the head. If we require less space around the head to carry out the procedure, you need a smaller area that’s fully sterile. This could be as simple as a laminar flow hood, as opposed to an OR. If that is the case, then a hospital could potentially save a lot of money with regard to infrastructure. You might even come to a point where, like dentistry, almost no ophthalmic surgical procedures are performed in an OR. Why would you require one, when most procedures are robotic-assisted microsurgeries, constrained to a small part of the human anatomy and performed under local anesthesia? With laminar flow hoods providing a constant supply of sterile air, operations could be performed in a surgical center or a day care facility, and I think in many ways this will change the approach to surgery. In principle, there’s no reason why you couldn’t use the same robot for multiple types of surgery. You may have to position the robotic arm differently for different types of procedures – like cataract, retinal or glaucoma surgery – but in principle, as long as you have appropriate access, the right algorithm, and the right protocol in place, then why wouldn’t you?
Asimov had his three laws of robotics; Hippocrates had his oath. Surgical robots are already being used routinely for laparoscopic procedures in general surgery and, for the most part, have successfully combined both Asimov’s laws and Hippocrates’ edict. Robotic surgery is no longer science fiction for general surgeons, and will soon become a reality for ophthalmic surgeons as well. Add in the fact that intraoperative imaging means that these robots can “see”, you can easily envisage automation of intravitreal injections that are consistently placed in the optimal location based on OCT data. I believe that soon, robots will automate multiple steps of routine, high-volume procedures like those involved in cataract surgery – and bringing with it significant cost savings too. But the fact is that these robots will do more than eliminate drudgery – they will enable some of the most complicated of cases to be performed in a safer and more reproducible manner, with better outcomes for patients. As the essence of both Asimov’s first law of robotics, and Hippocrates’ oath is to do no harm to humans, it’s gratifying to see that robots will go far beyond that and actively help many.
Marc de Smet is Director of MIOS, Lausanne, Switzerland (www.retina-uveitis.eu), and Medical Director of Preceyes Medical Robotics (www.preceyes.nl), Eindhoven, The Netherlands.
References
- M Spitznas, “Motorized teleguided stereotactic micromanipulator for vitreous microsurgery”, Arch Ophthalmol., 101, 623–630 (1983). PMID: 6838423. J Sillanpää et al., “A new table for work with a microscope, a solution to ergonomic problems”, Appl Ergon, 34, 621–628 (2003). PMID: 14559423. A Chatterjee et al., “Back pain in ophthalmologists”, Eye (Lond), 8, 473–474 (1994). PMID: 7821477. S Waqar et al., “Assessment of fatigue in intraocular surgery: analysis using a virtual reality simulator”, Graefes Arch Clin Exp Ophthalmol, 249, 77–81 (2011). PMID: 20890612. MD de Smet et al., “Comparison of robotic assisted ab externo sub-retinal bleb formation to manually performed surgery”. Poster presented at the 29th Meeting of the Club Jules Gonin; September 3–6, 2014; Zürich, Switzerland.
