Michael Korenfeld is the President of the Comprehensive Eye Care in Washington, Missouri, USA, and his practice is as wide ranging as its name suggests. Korenfeld has a special interest in research and innovation, and his practice took part in the clinical trials of Upneeq® (oxymetazoline hydrochloride ophthalmic solution), 0.1% from RVL Pharmaceuticals, a therapy for acquired ptosis in adults, which was approved by the FDA in 2020.
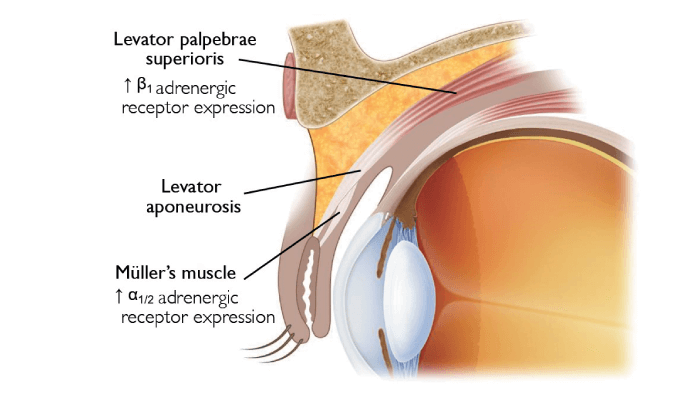
The pathophysiology of ptosis is not always the same. In the presence of ptosis, consideration of potential neurologic or orbital disease is important. Acquired ptosis is often caused by a weakness or stretching of the muscles lifting the eyelid and is broadly recognized as being among the most common disorders of the eyelid encountered in the clinic (1). Its prevalence increases with age, but ocular surgery is also a known risk factor (2). Upneeq activates the α-adrenergic receptors in Müller’s muscle, stimulating upper eyelids to contract and elevate (3, 4, 5).
Korenfeld describes the clinical trials for Upneeq that he took part in: “Various tests were used to measure how open patients’ eyelids were, down to tenths of a millimeter, with assessments on superior peripheral vision, which can be impaired and functionally troubling for people with ptosis; so both the appearance and visual function were tested before and after administering the medication to the treatment group.” A once-daily dose of Upneeq used for 42 days was found to significantly improve both the superior visual field and eyelid elevation with an average eyelid lift of 1.0 mm (6).
Korenfeld’s patients range from mild to severe stages of ptosis, although he points out that most people in mild stages will experience better and worse days, and just like with other muscles in their body, their levator function will fluctuate depending on the body’s energy reserves. In moderate stages of the condition, the muscle is so dysfunctional that even on good days the eyelid tends to droop, and in the most severe stages, some patients won’t be able to open their eyes at all. He comments: “What’s great about Upneeq is that patients with mild to moderate ptosis can use the therapy on the days they want to; for some it might be every day, for others – only on weekends or on special occasions.” The drop starts working quickly (often within 15 minutes) and the effect lasts for at least six hours. Before the drug became available, clinicians had to rely on a tricky and invasive surgical procedure, shortening the levator muscle with the creation of a pleat. The option to use Upneeq for some patients provides the clinician an effective alternative to this surgical procedure. Nowadays, Korenfeld regularly discusses acquired ptosis with his patients and offers appropriate candidates the option to trial a sample of Upneeq, with his office then handling the prescriptions that follow.
Acquired ptosis is a comorbid condition, which can impede a clinician’s access to the cornea and ability to get measurements used to make clinical decisions. This is why Korenfeld has also started discussing acquired ptosis with patients during an examination, when a patient has trouble opening their eyes sufficiently, or has acquired ptosis after years of prostaglandin use to manage glaucoma. “Recognizing that my glaucoma or cataract patients sometimes also suffer from acquired ptosis, and treating them, has made a great difference to my practice,” says Korenfeld. “Just two days ago, I had to measure the corneal curvature of a cataract surgery patient, and he couldn’t open his eyes effectively. In the past for a patient like this, I would’ve just reached around and held his eyes open or taped the eyelids, taken measurements and continued my exam without discussing ptosis with the patient. A situation like this has now become a trigger to treat and I take a moment to explain to the patient why their eyelids are drooping into their field of vision. I let them know that there is an eye drop that I want to try to see if it helps them. The patient is administered Upneeq, and after the examination these satisfied patients leave my practice with a prescription.”
“When I prescribe Upneeq I also make sure to tell my patients that they may have some adverse reactions and that in the clinical trials, the most common treatment emergent adverse reactions which occurred with an incidence 1 to 5% were: punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation and headache. I also keep in mind that Upneeq may not be right for all patients. Since Upneeq may impact blood pressure, I tell my patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to monitor their condition and seek medical care if it worsens.”
Has his evaluation and management of patients changed since the approval of Upneeq? “Now, when I diagnose a patient with acquired ptosis, I can prescribe them the drug, which creates a more complex interaction. It’s a win-win situation, as the visit is of a higher value to the patient, who can now address their condition.”
Korenfeld has also received positive reactions from patients: “Many patients who have been using Upneeq are telling me how great it is. Given that I usually see patients annually and that Upneeq was approved relatively recently, I expect these numbers to increase greatly in the coming months. The patients from clinical trials, who I of course saw on a more regular basis, were very happy to have been enrolled. They were able to notice a positive change in the appearance of their eyelids and that they looked more rested, with their friends commenting on the positive change in the appearance of their eyelids. Some patients mentioned how they were able to resume certain activities, such as reading in bed, which was much more difficult before they started the therapy.”
What about the rather unusual distribution model that RVL Pharmaceuticals has decided on? Korenfeld is a fan: “If I decide that a patient would benefit from using Upneeq, I give them some samples, and send an electronic prescription to RVL, who ships it directly to the patient. RVL therefore doesn’t have the burden to keep stock in every local pharmacy. The patient pays for Upneeq directly outside of the insurance process. I think it’s fantastic, and I wish more companies would use a similar model; it’s time and cost efficient for the physicians, office staff, and patients.”
To other ophthalmologists, he offers these words of wisdom: “I always tell my patients what I can about the drugs I prescribe for them: potential side effects and warnings, what it does, who it is for, what the onset of action is, and how long it lasts. For this purpose, I made a three-minute video that explains these things about Upneeq along with a transcript that the patient gets to take home. I then take any of their questions afterwards. It saves me a lot of time. As I do for all ocular pharmaceuticals, I make sure to tell my Upneeq patients to close their eyes for three minutes after administration, without blinking, so it has time to soak into the eye tissues, and not just stay in the conjunctiva. I also remind patients not to touch the tip of the container to their eye or any other surface to avoid contamination.” Korenfeld concludes: “I have seen this drug have a positive impact for many patients, and I really appreciate the fact that the patients can see the results for themselves, rather than simply taking my word for it.”
Dr. Michael Korenfeld is a paid consultant of RVL Pharmaceuticals, Inc.
IMPORTANT SAFETY INFORMATION
INDICATION
UPNEEQTM (oxymetazoline hydrochloride ophthalmic solution), 0.1% is indicated for the treatment of acquired blepharoptosis in adults.
WARNINGS AND PRECAUTIONS
• Ptosis may be associated with neurologic or orbital diseases such as stroke and/or cerebral aneurysm, Horner syndrome, myasthenia gravis, external ophthalmoplegia, orbital infection and orbital masses. Consideration should be given to these conditions in the presence of ptosis with decreased levator muscle function and/or other neurologic signs.
• Alpha-adrenergic agonists as a class may impact blood pressure. Advise UPNEEQ patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens.
• Use UPNEEQ with caution in patients with cerebral or coronary insufficiency or Sjögren’s syndrome. Advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop.
• UPNEEQ may increase the risk of angle closure glaucoma in patients with untreated narrow-angle glaucoma. Advise patients to seek immediate medical care if signs and symptoms of acute narrowangle glaucoma develop.
• Patients should not touch the tip of the single patient-use container to their eye or to any surface, in order to avoid eye injury or contamination of the solution.
ADVERSE REACTIONS
Adverse reactions that occurred in 1-5% of subjects treated with UPNEEQ were punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation and headache.
DRUG INTERACTIONS
• Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as beta-blockers, anti-hypertensives, and/or cardiac glycosides is advised. Caution should also be exercised in patients receiving alpha adrenergic receptor antagonists such as in the treatment of cardiovascular disease, or benign prostatic hypertrophy.
• Caution is advised in patients taking monoamine oxidase inhibitors which can affect the metabolism and uptake of circulating amines.
©2021 RVL Pharmaceuticals, Inc.
Upneeq® is a registered trademark of RVL Pharmaceuticals, Inc.
All rights reserved.
References
- J Bacharach et al., “A review of acquired blepharoptosis: prevalence, diagnosis, and current treatment options,” Eye (Lond) [Online ahead of print] (2021). PMID: 33927356.
- Y Wang et al., “Incidence and risk of ptosis following ocular surgery: a systematic review and meta-analysis,” Graefes Arch Clin Exp Ophthalmol, 257, 394 (2018). PMID: 30203103.
- B Esmaeli-Gutstein et al., "Distribution of adrenergic receptor subtypes in the retractor muscles of the upper eyelid,” Ophthalmic Plast Reconstr Surg, 15, 92 (1999). PMID: 10189635.
- BC Skibell et al., “Adrenergic receptors in the ptotic human eyelid: correlation with phenylephrine testing and surgical success in ptosis repair,” Ophthalmic Plast Reconstr Surg, 23, 367 (2007). PMID: 17881986.
- SJ Park et al., “Distribution of adrenergic receptor subtypes and responses to topical 0.5% apraclonidine in patients with blepharoptosis,” Ophthalmic Plast Reconstr Surg, 34, 547 (2018). PMID: 29634605.
- CB Slonim et al., “Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis,” JAMA Ophthalmol, 138, 1168 (2020). PMID: 33001144.
