
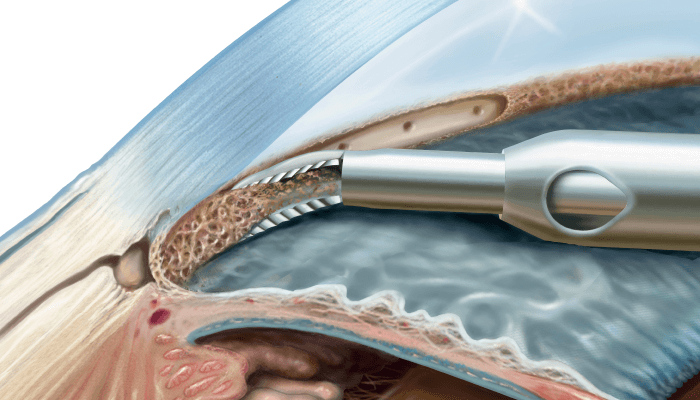
The number of glaucoma patients is expected to exceed 88 million by the end of 2019 (1); the need for minimally invasive surgical glaucoma treatments has never been greater. Fortunately, pioneering surgical company MST has the answer: a range of excisional goniotomy devices. Commercially available in over 56 countries, the MST Excisional Goniotomy portfolio consists of the Trabectome and TrabEx product lines, designed to increase aqueous outflow and promote enduring intraocular pressure (IOP) reduction through the removal of diseased trabecular meshwork (TM)*. These devices facilitate complete edge-to-edge TM removal, providing lasting exposure of collector channels.
TrabEx boasts laser-sharpened, serrated blades that have been precision-engineered to cut, rather than tear, to facilitate safe, efficacious TM removal, while TrabEx+ adds the additional benefit of I/A for increased visibility during surgery. The Trapezoidal shape of the TrabEx blades, customize the excision to varying patient anatomies and TM’s.
Notably, Trabectome uses electro-surgical ablation, rather than cautery, to completely fragment the diseased TM. All three devices also feature a rounded heel that guides the devices around the anatomical arch of Schlemm’s Canal.
“The MST excisional goniotomy devices are the premier option for minimally invasive surgical glaucoma (MIGS) treatment,” states Erik Bonn, Director of Commercial Development at MST. “These devices are indicated for a range of clinical scenarios: the management of pediatric or adult mild, moderate, or severe glaucoma; open or narrow angle glaucoma; primary or secondary glaucoma; as well as pseudoexfoliative or non-pseudoexfoliative glaucoma. This allows users of Trabectome and TrabEx to treat a wide range of patients.”
How does that translate to the clinic? According to data collected over seven years – very well. Trabectome has proved to be a highly efficacious and easy-to-use surgical platform; clinical studies show that a significant majority of patients who had Trabectome surgery experienced a sustained IOP reduction to ≤21 mmHg – a 20 percent improvement from the IOP baseline – with no need for additional glaucoma surgery (2). The knock-on effect is that the Trabectome also results in a significant reduction in glaucoma medication use, which helps negate patient compliance issues (2).
MST’s desire to improve on the results of existing MIGS devices has paid off. In its excisional goniotomy line, MST has provided a premium offering for the removal of diseased TM. By simplifying excisional goniotomy, Trabectome, TrabEx, and TrabEx+ make it easier for glaucoma and cataract surgeons alike to treat a broad range of patients – without the need for more invasive procedures (such as trabeculectomies and aqueous shunts). MST will continue to innovate in the glaucoma space and extend its complex surgery offering, delivering on its promise to improve vision for patients worldwide.
For more information visit microsurgical.com.
* TrabEx and TrabEx+ were formerly known as “Goniotome” and “Goniotome + I/A.” Their product names are scheduled to take immediate effect in the US and April 2020 in Europe.
References
- Market Scope, "Global Glaucoma Surgical Device Market report" (2015).
- S Mosaed, “The first decade of global trabectome outcomes”, European Ophthalmic Review, 8, 113 (2014).
