VEGF is something of a Jekyll and Hyde molecule, with both physiological as well as pathological functions (1). Although critical for vascular development, it’s also strongly linked to angiogenic retinal pathologies, such as wet AMD and diabetic macular edema. Intravitreal anti-VEGF injections are the gold standard for treating the choroidal neovascularization that’s associated with such diseases, and although they have transformed patients’ outcomes, they aren’t a panacea. In many cases, their efficacy does not last forever, and there is concern that VEGF inhibition in the retina may lead to thinning and atrophy of the choriocapillaris, photoreceptor degeneration, eventually causing damage to the retina – the complete opposite of the intended result. Accordingly, researchers continue to seek a better therapy – but finding one may require a greater understanding of VEGF’s role in ocular pathologies, according to Alexander Marneros, of Massachusetts General Hospital. Marneros used a genetically modified mouse model which expresses VEGF-A at two-to-three times physiological levels, with VEGF expression found in the lens, retina and ciliary body, in order to study the protein’s role in AMD pathogenesis. “Identifying the downstream mechanisms by which VEGF-A influences the development of these diseases may lead to new therapies that don’t have the adverse effects of blocking VEGF-A itself,” he says.
In his pursuit of new knowledge, Marneros and his colleagues made an interesting discovery – as well as uncovering further evidence of the role of VEGF-A in both wet and dry macular degeneration, he discovered a link to another, incredibly common ocular pathology: “We found that increased VEGF-A in the lens is associated with increased oxidative stress and cataract formation. This finding provides the first evidence for a role of increased VEGF-A in the development of age-related cataracts. Notably, we found that VEGF-A is expressed throughout life in cells of the lens. Whether VEGF-A plays any roles in the aging processes of the lens has been completely unknown, but our experiments provide the rationale for future studies into a possible link with cataract formation.” Oxidative stress and the production of reactive oxygen species (ROS) seem to play a pivotal role in AMD pathogenesis, and as VEGF-A is known to induce the production of ROS, it’s possible that VEGF plays an important role in this cycle. It is generally thought that hypoxia and oxidative stress may drive VEGF production, which in turn leads to additional oxidative stress, and eventually to the development of pathologies such as cataract and AMD (2).
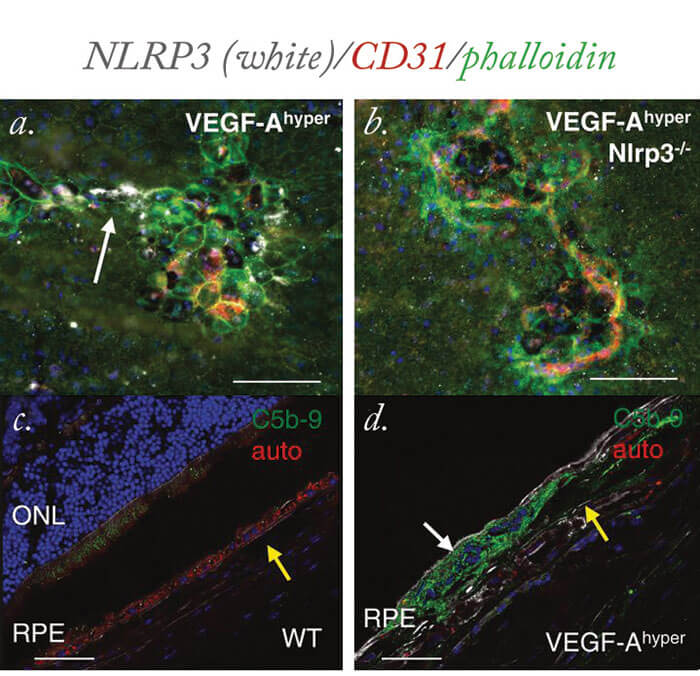
But how can these findings point towards potential new therapies? Tissue damage resulting from oxidative stress is associated with activation of the NLRP3 inflammasome, and expression of the proinflammatory cytokines IL-1β and IL-18 (2). One encouraging finding was that inhibition of either IL-1β or the NLRP3 inflammasome may inhibit the pathological effects of increased VEGF-A, says Marneros (see Figure 1). He believes that future clinical studies in patients would be essential to assess the role of inhibitors of IL-1β or the NLRP3 inflammasome in preventing or inhibiting AMD and senile cataracts, while maintaining the beneficial effects of normal levels of VEGF-A, which is required for the normal function of the retina and choroidal vasculature. “Our studies also provide the basis for future experiments to assess links in the pathogenesis of AMD and cataracts in patients,” says Marneros.
References
- M Saint-Geniez, P D’Amore, “VEGF has physiological as well as pathological functions”, The Ophthalmologist, 4, 16–21 (2014). Available at: http://bit.ly/1qLg75Y. AG Marneros, “Increased VEGF-A promotes multiple distinct aging disease of the eye through shared pathomechanisms”, EMBO Mol Med, 8, 208–231 (2016). PMID: 26912740.
