Professor and Former Chairman at the UST Hospital Eye Institute, Chief of the Lacrimal, Orbital and Oculofacial Plastic Surgery Section at the University of Santo Tomas Hospital, University of Santo Tomas Espana in Sampaloc, Manila, Philippines,specializes in lacrimal, orbital, and oculofacial plastic surgery. Javate is Past President of the Philippine Society of Ophthalmic Plastic and Reconstructive Surgery; Founding President of the Asia-Pacific Society of Ophthalmic Plastic and Reconstructive Surgery; President of 10th International Society of Dacryology and Dry Eye; and Fellow of the American Society of Ophthalmic Plastic and Reconstructive Surgery.
Here, he shares his experience with the Ezypor® orbital implant from FCI S.A.S. (Paris – France), which can be used after enucleation or during secondary orbital implant surgery.
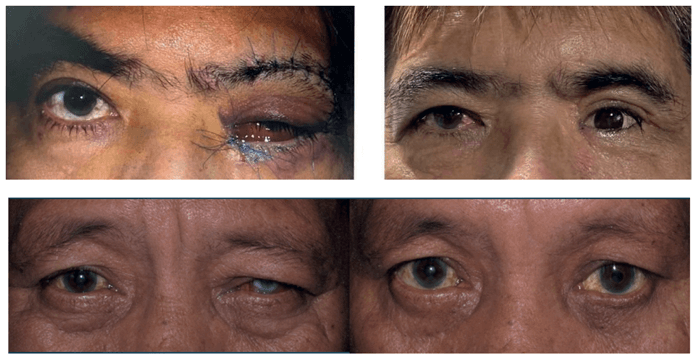
Management of the anophthalmic socket is challenging, and a decision on implant selection and wrapping material should ideally be made during pre-operative planning. Orbital implants are used to replace the orbital volume and allow some amount of realistic movement of a prosthetic eye. Porous orbital implants are most commonly used to fill the anophthalmic socket following enucleation, but despite a variety of these implants being available on the market, specialists still see patients with complications following implantation. This is why a new, reliable orbital implant has been designed and manufactured by FCI S.A.S., to increase tolerability, make the procedure easier and more time-efficient for surgeons, and improve patient outcomes.
Porosity matters
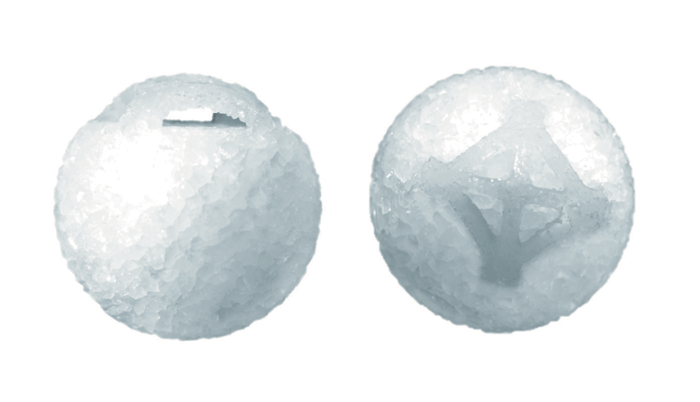
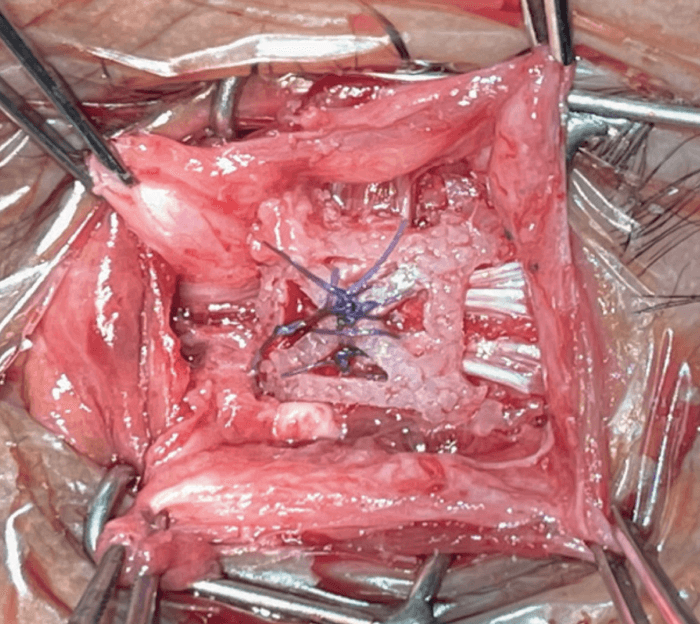
This innovative product – the Ezypor® orbital implant – is made of Ultra High Molecular Weight PolyEthylene and has a good porosity structure. It features an innovative and patented smooth anterior suturing platform that enables the extraocular muscles to be sutured directly onto the implant. It is generally well tolerated in the anophthalmic socket, without the need for wrapping material or an autologous graft. Additionally, it offers multiple suturing options to suit different surgeons’ preferences.
Ezypor®’s porosity is between 40 percent and 60 percent, as it is designed for optimum colonization of fibrovascular tissue – a good base for fibroblast integration. The implant has eight suture channels to facilitate needle insertion and muscles fixation that allow easy attachment to the extraocular muscles.
Smoother surface
Why choose an orbital implant with a larger pore size? This type of implant demonstrates better fibrovascular ingrowth, and I have observed increased frequency of spontaneous healing of exposures, whenever they occur. Other porous polyethylene structures have relatively rough surfaces that may promote breakdown of the overlying tissue by mechanical pressure, and friction between the prosthesis and anterior surface of the implant, leading to anterior exposure. That’s why Ezypor®, with its smooth surface tunnel porous polyethylene, is such a key innovation in the field of orbital surgery.
Conclusion
To summarize: why do I choose Ezypor® in my practice? The implant’s smoother anterior surface minimizes the rubbing effect against the tissues to decrease the risk of implant exposure. It has a good porosity structure, which is a good base for fibroblast integration, and it is less abrasive than other porous implants. Importantly, the cost and time of the surgery is reduced as the implant doesn’t need wrapping material. With better tolerability and fewer complications, this is my orbital implant of choice.
FCI S.A.S. is committed to fostering a close relationship with key opinion leaders in the company’s two strategic fields: oculoplasty and vitreoretinal surgery. Since the company’s creation in 1984, significant resources have been directed to R&D, making FCI a world leader in its two specialties. Despite a significantly reinforced regulatory environment, innovation is part of FCI’s DNA. This will help us shape the future leading solutions in oculoplasty and vitreoretinal procedures.
Ezypor® is both CE-marked for use in the EU, and FDA-approved for use in the USA.