Despite being a rare disorder, retinitis pigmentosa (RP) is the leading cause of visual disability and blindness in patients under 60 years old (1). The primary focus of ongoing research into potential treatments for RP has been on gene and stem cell therapy. And though advancements are being made, these therapies are often invasive and may not be appropriate for routine clinical management.
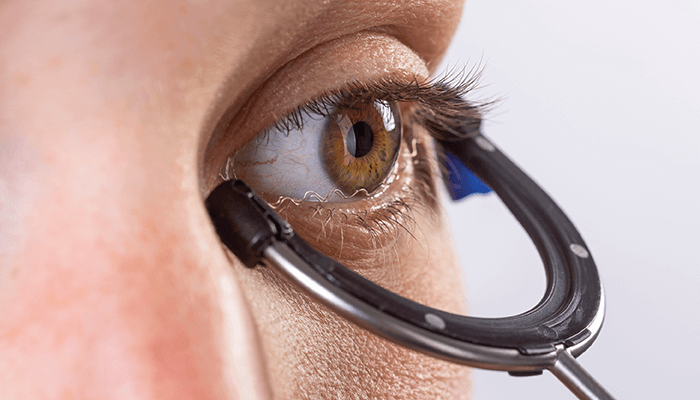
Transcorneal electrical stimulation (TES) therapy – developed by Okuvision – offers an alternative treatment route. Non-invasive in nature, the treatment involves placing an electrode on the lower lid that contacts the eyeball at the inferior corneal limbus before electrical impulses – maximum 1 mA and 20 Hz – occur over 30 minutes. After brief physician-led instruction and training, patients can perform the therapy themselves once a week.
A recent analysis of a previous clinical study – carried out by ophthalmologists from the University Eye Clinic Tübingen and the Eye Clinic of Stuttgart Medical Center – has provided compelling evidence for the effectiveness of this therapy (2). In the study, 32 patients received weekly TES treatment and 20 patients received sham treatment. After a year, eyes that had undergone TES displayed a visual field loss of 2.1 percent, while untreated contralateral eyes had an average visual field progression of 5.8 percent. In contrast, the placebo group experienced an annual decline of 7.5 percent. Consequently, the reduction in visual field area measured by kinetic perimetry was a remarkable 64 percent less in the eyes treated with TES than in the untreated eyes (p=0.013) and 72 percent less than in the placebo group (p=0.103).
Here, we speak with Alfred Stett, CEO of Okuvision, who explores the details of the new analysis, his hopes for the future of the therapy, and the all important issue of reimbursement.
How does this new analysis expand our understanding of TES treatment?
The new analysis was exploratory. It has revealed a previously unrecognized dose-dependent effect of TES. In studies so far, a multiple of the individual threshold of perception for electrically evoked light flashes (EPT) has been used to define the individual stimulation strength. As phosphene thresholds are relative and cover a wide range of values, the current strengths were also very different from one individual to the next. By comparing EPT-related study groups (for example, 150% EPT versus 200% EPT), the current-dependent effect of TES remained obscured (3). The new analysis shows us that it is the intensity of electrical stimulation that makes the difference, not the subjective electrical phosphene thresholds (EPT).

What does OkuStim mean for the future of RP treatment?
There will be no cure for RP in the foreseeable future. The question of whether it is possible to develop a therapy that can stop the disease or reverse the loss of vision remains unanswered.
Though OkuStim may not be a cure, it offers patients the chance to slow down the loss of the visual field and, in the best case scenario, maintains vision for a long time without serious impairments in everyday life, pushing back the onset of legal blindness by significant years. Moreover, slowing down the progression of RP is beneficial for future treatment options, including gene and stem cell therapies, which require the retention of sufficient retinal cells and cellular connections.
Based on several clinical trials that demonstrate its beneficial effects and safe usage (4), TES therapy using the OkuStim system is already available today in Germany, Switzerland, Austria, Italy, France, the UK, Turkey, Greece, and Norway. More countries will be added, including ones outside of Europe.
Can the therapy be improved?
Two approaches offer the potential for improving TES therapy: i) Dose and delivery form, and ii) Mechanism of action.
As with all treatments, the delivery form, dose, and frequency of administration are important factors that influence therapeutic effect. The delivery method is transcorneal stimulation with a specific temporal pattern. The pattern or waveform today consists of biphasic current pulses with a pulse duration of 10 ms and a frequency of 20 Hz. This waveform has remained unchanged since the first clinical study, and the weekly application for 30 minutes is also a fixed component of the approved therapy. Only the amplitude of the current pulses is dosed patient-specifically. Depending on the stage of the degenerative retinal disease or genetic cause, it is conceivable that different waveforms and dosages may be more effective for some patients.
Fundamentally, to further develop the therapy in a targeted way, the mechanism of how neuroprotection and clinical benefit emerge from electrical stimulation of the eye must be further researched and understood. There are still large knowledge gaps here – even though numerous preclinical studies show that electrical stimulation influences inflammatory and apoptotic signaling pathways in retinal cells.
What about reimbursement?
Our goal is to make this therapy available to all patients but, to do this, we need to tackle reimbursement. TES therapy is not yet regularly reimbursed by health insurance companies so patients usually have to pay for it out of their own pockets.
Although the German Ophthalmological Society has issued an updated guideline in 2021 recommending electrostimulation as a therapy for RP (5), TES is not yet recommended as an evidence-based treatment option for RP in national and international guidelines. Both regular reimbursement and recommendation in guidelines require further proof of therapeutic benefit over a longer observation period. Fortunately, the German Federal Joint Committee (G-BA) has commissioned a manufacturer-independent trial to clarify whether TES has patient-relevant benefits in long-term application (6). The multicenter study has enrolled more than 134 patients with RP in 17 German eye hospitals who will trial TES-Therapy for a period of three years. Enrollment closed in February of this year – and we are hoping for a positive benefit decision in 2026.

References
- N Cross et al., “Retinitis Pigmentosa: Burden of Disease and Current Unmet Needs,” Clin Ophthalmol, 16, 1993 (2022). PMID: 35757022.
- Okuvision, “New evidence of efficacy of transcorneal electrical stimulation (TES)” (2023). Available at: http://bit.ly/3ZiGQI9.
- A Stett et al., “Transcorneal Electrical Stimulation Dose-Dependently Slows the Visual Field Loss in Retinitis Pigmentosa,” Transl Vis Sci Technol, 12, 29 (2023). PMID: 36809335.
- N S Kahraman, A Oner, “Effect of Transcorneal Electrical Stimulation on Patients with Retinitis Pigmentosa,” J Ocul Pharmacol Ther, 36, 609 (2020). PMID: 32429728.
- AWMF online, “Therapie und Hilfeleistungen” (2021). Available at: http://bit.ly/3J1SsZd.
- Gemeinsamer Bundesausschuss, “TES-RP – Transkorneale Elektrostimulation bei Retinopathia Pigmentosa” (2021). Available at: http://bit.ly/3L4s2Zm.
