“We hope to be able to provide a new method of treating patients with age-related macular degeneration (AMD),” says Felicity de Cogan of the University of Birmingham, UK, and lead author on a recently published paper describing topical delivery of anti-VEGF antibodies to the posterior segment (1). And it looks like they may have found one – with the help of cell-penetrating peptides (CPPs). Originally, de Cogan was researching the use of CPPs in microbiology, but through collaborations with neuroscientists at her institute (Lisa Hill and Ann Logan) and clinicians from Queens University, Belfast, UK (Mei Chen and Heping Xu), the potential of CPPs to deliver drugs into the eye became evident. “Anti-VEGF therapies are well-established treatments for AMD but there has been little research on their topical delivery,” says de Cogan. “The CPP formulation brings together the well-established field of CPPs and the unmet clinical need for improved delivery methods for patients with AMD.”
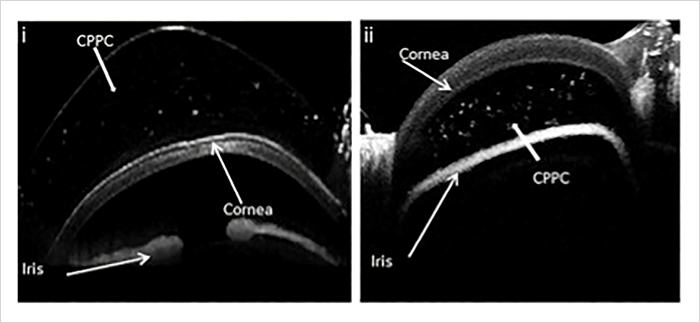
CPPs (also known as protein transduction domains) act as chaperones, facilitating the cellular uptake of complexed proteins, but according to the authors, “The internalization mechanism has not been fully elucidated.” In their study, the team exploited simple charge-based interactions to formulate anti-VEGF drug complexes decorated by the CPPs. After confirming that the peptides were nontoxic to cultured ocular cells, they topically applied complexes of CPPs and anti-VEGF drugs (bevacizumab or ranibizumab) to rat and pig eyes. Through OCT imaging and enzyme-linked immunosorbent assay analysis of ocular tissues, they demonstrated that CPPs were able to enter the anterior chamber (Figure 1) and reach the posterior segment. “We were surprised at how quickly CPPs could enter the eye after drop application; they can be seen in high quantities in only six minutes,” says de Cogan, adding that a key finding was the fact that CPPs could carry a large therapeutic protein such as anti-VEGF through to the back of the eye.
The team also compared anti-VEGFs delivered by CPPs with intravitreal injection in a mouse model of choroidal neovascularization (CNV), and found them equally efficacious at reducing lesion size (both p<0.001 versus negative controls of CPP-only, anti-VEGF-only and PBS eye drops). “The anti-VEGF therapeutic had the same outcome on disease progression whether it was delivered topically by CPPs or intravitreal injection,” says de Cogan. The topically-delivered anti-VEGFs were found to reach physiologically-relevant concentrations and clearance from the retina after 24 hours suggested the need for a daily dosing regimen. According to de Cogan, CPPs are highly effective antimicrobial agents, potentially negating the need for preservatives in eyedrop formulations. But what about the issue of poor patient adherence to eyedrop regimens? “The regime won’t work for everyone and some patients may find a monthly injection preferable. The key to this technology is that it gives patients a choice and provides an alternative that might have reduced side effects,” says de Cogan. The team hope to begin clinical trials within a year of raising funding.
References
- F de Cogan et al., “Topical delivery of anti-VEGF drugs to the ocular posterior segment using cell-penetrating peptides”, Invest Ophthalmol Vis Sci, 58, 2578–2590 (2017). PMID: 28494491.
