- AMD represents a significant public global health burden, but is only typically diagnosed when patients have intermediate to advanced stages of disease
- Dark adaptation, the ability of the eye to adjust from light conditions to dark ones, can be impaired in patients with AMD and other retinal conditions
- Historically, the use of dark adaptometry to diagnose AMD has been limited thanks to lengthy and burdensome tests – but faster tests are now becoming available
- Identifying patients with impaired dark adaption could have a beneficial impact on early AMD diagnosis and management.
Age-related macular degeneration (AMD) represents a significant public health burden; over eight percent of the global population live with this chronic condition, and are at risk of progressive central vision loss (1). The disease is typically first diagnosed in its intermediate to advanced stages through fundus examination, and autofluorescence and OCT imaging. However, there is evidence suggesting that very early functional changes can occur – possibly even before structural changes in the fundus can be detected, implying that AMD could be diagnosed much earlier than it is now. Earlier diagnosis could be achieved through functional methods alone or in combination with imaging modalities, and would have important implications for how the disease is managed, as well as patients’ prognoses. Because patients with AMD commonly complain of impaired night vision, assessing dark adaption might be one such method.
The evolution of dark adaptometry
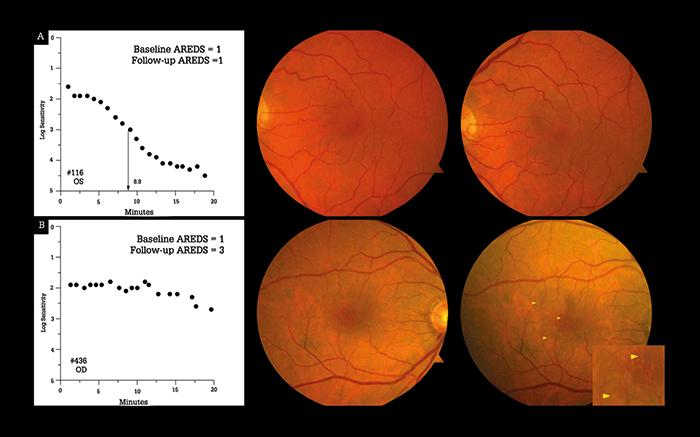
Dark adaptation refers to the ability of the eye to transition from well-lit or bright environments to dark or dim ones, and is measured through dark adaptometry (See Box – Dark Adaptometry 101). Though the field of medical retina has exploded in terms of volume and sophistication when it comes to diagnostic imaging techniques and treatments, dark adaptometry and assessments to measure and monitor partial night blindness have been ‘left in the dark.’ In the past, measuring dark adaptation had found few uses in the clinic, as it was considered to be relevant for diseases such as retinitis pigmentosa and vitamin A deficiency – and these can be easily identified by other means. Additionally, traditional dark adaptometers, such as the Goldmann-Weekers device, are manually operated and testing can take 60–120 minutes in a dark room, which can be very tiresome for both the patient and the operator. Consequently, there are few manual dark adaptometers around and possibly even fewer people who are trained to use them. To address the issue, rapid adaptometers and methods have been proposed, one of which is the AdaptDx (Maculogix) – a dark adaptometer that has been optimized for the assessment of partial night blindness in AMD.Dark adaptometry measures the absolute thresholds of cone and rod sensitivity. In the traditional testing format, the patient (under dark conditions) is presented with flashes of light that cause photobleaching and eliminate all or some of the patient’s dark adaptation. The patient’s rate of dark adaptation is measured by measuring their response to a dynamic stimulus – after the flash, the dark adaptometer measures the lowest intensity of light that the patient can see (the threshold light intensity) as a function of time and shows it as a dark adaptation curve (Figure 1). The intensity of illumination needed for a flash of light to be just visible decreases with time in a biphasic pattern if the patient has normal vision. The initial phase of adaptation is mediated by the cone photoreceptors, which are responsible for daylight vision, after which follows a phase of adaptation mediated by the rod photoreceptors, which enable night vision. Traditional dark adaptometers include the Goldmann-Weekers, Roland, Metro-vision and YAK-II devices. The rapid dark adaptometry device, AdaptDx, is a short-duration dark adaptation protocol that induces photobleaching through a short, intense flash, followed by immediate measurements of patient sensitivity to light stimulus. Patients are asked to indicate when a fixation light is visible to them, and with each response, the fixation light decreases in intensity. RI represents when recovery of visual sensitivity and dark adaptation is completely mediated by rod photoreceptors.
Originally developed for use in research settings (such as clinical trials) where optimizing speed, accuracy, and comfort are essential to data acquisition and patient retention, the AdaptDx can perform exams in as little as 6.5 minutes, and can measure rod intercept (RI), an estimate of rod recovery speed that correlates closely with data acquired from traditionally used adaptometer models (2).
Bringing things to light
How can measuring RI help patients with AMD? Studies have shown RI to be a sensitive and specific diagnostic parameter, both in identifying AMD and for predicting future disease. One study assessed both healthy individuals (n=21) and patients with confirmed AMD (n=127), and found that 90.6 percent of patients with AMD had an abnormal RI value – showing high sensitivity (p<0.001) – and that 90.5 percent of healthy patients had a normal RI value – showing high specificity (p<0.027) (3). When the severity of disease was assessed, the diagnostic sensitivities were 80.5 percent, 94.4 percent, and 100 percent for early, middle, and late AMD, respectively. In a prospective cohort study, RI was measured in 325 patients with normal macula health (both eyes were step 1 on the Age-related Eye Disease Classification system [AREDS]). At the three year follow-up, patients who had an abnormal RI value at baseline were twice as likely to have developed AMD compared with the rest of the study population (4)(Figure 1). These findings suggest that impaired dark adaptation might be predictive of AMD before structural lesions have even developed – a finding supported by other studies (3)(4)(5)(6). Together, these studies show that dark adaptation is impaired in AMD and suggest that delays in dark adaptation, as measured by the RI value, can predict future AMD and distinguish between early, middle, and late-stage AMD.Implications for clinical practice
Like many other retinal degenerations, AMD initially causes death of rod photoreceptors, ultimately leading to cone degeneration and loss of vision that greatly affect the ability of the patient to lead an independent life. If rod degeneration could be prevented, perhaps cone-based vision could be saved as well. Detecting the presence of disease earlier – or a patient’s potential to develop it – would, therefore, be preferable. Dark adaptation is an important aspect of retinal function that could aid earlier treatment intervention, as well as earlier monitoring of disease progression for research purposes and clinical practice alike. And that’s why dark adaptometry is now transitioning from the research setting into routine clinical care to diagnose and monitor AMD (7), as well as other conditions that can impair dark adaptation, such as congenital stationary night blindness, retinitis pigmentosa and vitamin A deficiency. Measuring dark adaptation as a functional test of retinal health (in combination with fundus photography and OCT) to assess a patient’s risk for developing AMD could also improve how ophthalmologists approach the condition. For example, if a seemingly healthy patient shows impaired dark adaptation but no other eye disease has been identified, the physician could encourage the patient to take preventive measures against AMD: stopping smoking and making dietary and behavioral changes, specifically, eating more vegetables, losing weight (if necessary), and doing more regular aerobic exercise. The fear of going blind has been found to be on a par with the fear of developing cancer or Alzheimer’s disease in patients in the US (8), so a predictive indicator of AMD could provide the impetus patients need to make lifestyle changes that prevent or delay the onset and progression of AMD. In my experience, patients are particularly motivated by personalized counseling from a health professional who can confirm and clarify the many recommendations aimed at the general public from sources that are not always reliable.The bigger picture
Just like high blood pressure and cholesterol are warning signs that motivate patients to discontinue bad habits and make lifestyle changes, impaired dark adaptation could be used as a tool to encourage patients to make overarching improvements in their life, not just for their ocular health, but for their overall wellbeing. By reinforcing this message, ophthalmologists can do more to contribute to general health, which will reduce the public health burden of not only AMD, but countless other diseases as well. Michael Larsen is Professor of Clinical Ophthalmology at the Rigshospitalet in Copenhagen and at the University of Copenhagen. Financial disclosures: Larsen reports that he has no financial interests in MacuLogix Inc.References
- WL Wong et al., “Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis”, Lancet Glob Health, e106–1016 (2014). PMID: 25104651. GQ Yang et al., “Recent advances in the dark adaptation investigations”, Int J Ophthalmol, 8, 11245–1252 (2015). PMID: 26682182. GR Jackson et al., “Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration”, Invest Ophthalmol Vis Sci, 55, 1427–1431 (2014). PMID: 24550363. C Owsley et al., “Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration”, Ophthalmology, 123, 344–351 (2016). PMID: 26522707. GR Jackson et al., “A short-duration dark adaptation protocol for assessment of age-related maculopathy”, J Ocul Biol Dis Infor, 1, 7–11 (2008). PMID 20072631. C Owsley et al., “Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health”, Curr Eye Res, 41, 266–272 (2016). PMID: 25802989. SK Holfort et al., “Dark adaptation during transient hyperglycemia in type 2 diabetes”, Exp Eye Res, 91, 710–714 (2010). PMID: 20732318. AW Scott et al., “Public attitudes about eye and vision health”, JAMA Ophthalmol, 134, 1111–1118 (2016). PMID: 27490785.
