- DMEK corneal graft dissection and anterior chamber placement methods are hard to learn and difficult to perform
- A simpler process, from dissection to placement, is proposed
- Visual outcomes are equivalent to other DMEK methods
- This technique avoids touching the endothelium during the dissection, which kills cells
The availability of corneal tissue, as with other organs for transplantation, relies on the goodwill of people to bequeath them for use after their death. Numbers are finite; this is a precious resource.
For a number of diseases, such as Fuchs’ dystrophy, bullous keratopathy (or the failure of previous penetrating keratoplasty), the current standard of care is endothelial keratoplasty, where the patient’s diseased corneal endothelium is replaced with an endothelial graft. Two major techniques for the preparation of such grafts have been developed: Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) and Descemet Membrane Endothelial Keratoplasty (DMEK). Although DSAEK is relatively simple to perform, the transplant retains the stroma and Descemet’s membrane, which can distort vision after implantation. DMEK utilizes only the endothelium from the donor cornea which, once grafted, reproducibly gives good visual outcomes. However, the preparation, manipulation and placement of a DMEK graft is particularly challenging to learn and perform.
I sometimes found it difficult to perform this surgery because of the risk of tearing the Descemet’s Membrane, when we have to hold the membrane with forceps. Furthermore, traditional DMEK is performed under balanced salt solution (BSS) – the “SCUBA” technique – which has one big problem: once the detachment has been performed, the graft always rolls up with the endothelium to the outside. That’s because every time you leave the tissue free in solution, it spontaneously does this. It makes placement of the graft within the anterior chamber much more difficult than if the endothelium was on the inside of the roll. It was clear to me that matters needed to be rethought.
Punch It Out
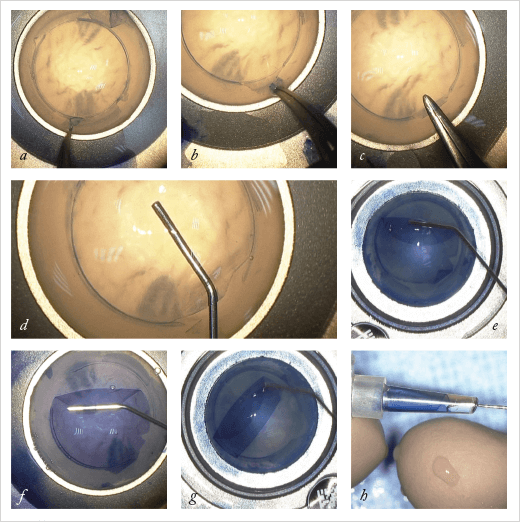
The first change that I made was to the circular trephination of the donor tissue. When I first started doing this dissection ten years ago, I did so by placing an air bubble in the stroma. This detached the tissue of interest from the endothelial-side components. Following this I performed circular trephination. I wanted to find the real plane between the Descemet’s membrane and the stroma, but it was difficult to obtain this planal dissection every time; the dissection was often within the stromal layers. Sometimes when I performed this superficial trephination it wasn’t successful over the entire 360°. However, far from being an issue, this incomplete trephination led to improvements. In the area where trephination was unsuccessful, if I could detach the Descemet’s membrane from right to left I had the plane of dissection outside the section; I could enter in the middle of the cornea very easily at this point. To do this deliberately, I simply broke the circular trephine’s blade over 30°. The technique was published in the American Journal of Ophthalmology in 2013 (1). However, homemade broken blades aren’t ideal for use in corneal surgery. Sometimes it’s difficult to break the circular blade to obtain the 330° section of the Descemet’s membrane. And when you are using a broken blade you can’t press too hard, in case you perforate the lot. So I wondered if it was possible to design a punch, where the blade was missing over 30° and where there was a stop at around 300 µm, so that even if it was pressed hard on the top of the endothelium, it didn’t perforate the entire circle. That would reproducibly, section the Descemet’s membrane at 330° (Figure 1).Sunny Side Up
Once punched, the donor cornea is placed endothelial side up in an artificial chamber, sealed, and the area that was punched is stained with Trypan blue (Figure 2). The graft is then protected by methylcellulose, and the Descemet’s membrane between trephination and limbus is peeled back with forceps, leaving an upside-down flap at the hinge (that is, the 30° region is not punched out). Manual dissection under the hinge flap is performed and is extended 1 mm into the graft area, initiating a delamination plane and permitting hydrodissection of the Descemet’s membrane until it is completely detached. This is a difference to the SCUBA approach. The dissection is not under BSS when we perform the separation of the Descemet’s membrane – at all times, it is in contact with the underlying stroma, which has implications later in the procedure. The hydrodissection has another advantage over the SCUBA technique; the surgeon is not peeling to separate the endothelium from the stroma at this point, reducing the risk of tearing.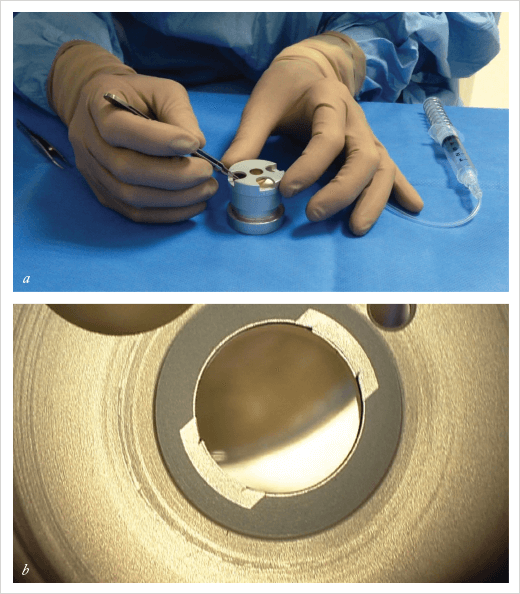
After protecting the membrane with a small amount of methylcellulose, the surgeon uses a 27 gauge cannula to separate the entire Descemet’s membrane and then to lift the membrane and fold to the center – into a burrito-like shape, with the endothelium to the inside of the burrito. All that’s then left to do is fill an intraocular lens (IOL) cartridge with corneal storage medium, place the membrane burrito into the cartridge, insert the graft into the anterior chamber with the endothelium facing the iris – much like you would do with a DSAEK graft – and inject BSS to unfold the graft, centering the graft with an air bubble to maintain it in position. This is the point where the approach to dissection pays off. When we unfold the roll, the endothelium is on the inside (Figure 3), because the section was not under BSS when the Descemet’s membrane is being separated. This means that it’s far easier to unfold the graft in the anterior chamber.
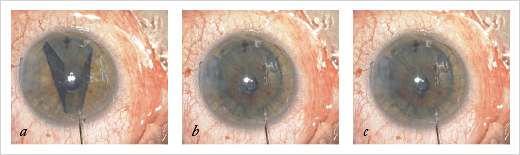
Avoiding Death Grips
Every time a surgeon touches the endothelium during the SCUBA technique, cells are lost. Using the dissection technique described above, the surgeon works underneath the Descemet membrane, never touching the endothelium. Theoretically, at least, this approach spares cells, resulting in more robust grafts. I have not personally performed any comparative studies but an American team from the Lions VisionGift facility in Portland, OR, USA compared the SCUBA technique and my system, and they found a difference. I prefer that others have done it because I may have a personal interest in such questions.Is Easier Better?
This technique is easier than regular DMEK. Visual recovery is the same, because with both techniques, you only have Descemet’s membrane and endothelial cells. Surgeons who are proficient in SCUBA-technique DMEK can routinely and reproducibly achieve similar surgical success rates as are achieved with my technique. However, it can take a long time to get there; the SCUBA technique learning process with technique is strenuous. I presented a paper at the European Society of Cataract and Refractive Surgeons meeting in Amsterdam in October 2013, reporting on when I asked two of my residents who had never performed DMEK or any similar dissection before to perform the corneal dissection using this new approach (2). I sat beside them and explained step-by-step how to perform the procedure, and in 14 out of 16 cases they successfully isolated the graft on their first dissection. This clearly demonstrates that it’s easier to do it with this system. One additional benefit of a simple, highly reproducible and easy-to-learn technique for DMEK graft preparation is that more ophthalmologists are likely to learn it. This increases the availability of a corneal graft procedure that provides excellent visual outcomes, but that has, to date, been limited to a small number of experienced surgeons. For those with corneal diseases like Fuchs’ dystrophy or bullous keratopathy, that’s great news.Marc Muraine is Professor of Ophthalmology at CHU de Rouen, Hôpital Charles Nicolle, Rouen, France.
References
- M. Muraine, J. Gueudry, Z. He, S. Piselli, et al., “Novel technique for the preparation of corneal grafts for Descemet membrane endothelial keratoplasty”, Am J Ophthalmol, 156(5), 851–9 (2013). M. Muraine, D. Toubeau, J. Gueudry, S. Lefevre, “Simple novel surgical device to facilitate preparation of endothelial grafts for DMEK”. Free paper presentation, presented at the XXXI Congress of the European Society of Cataract & Refractive Surgeons, Amsterdam, October 8th, 2013.
