
Please see Full Prescribing Information for DEXYCU.
What can you tell us about DEXYCU?
DEXYCU® (dexamethasone intraocular suspension) 9% is the first and only FDA-approved, single-dose, sustained-release, intracameral steroid for the treatment of cataract postoperative inflammation (1, 2, 3). It is administered at the end of cataract surgery, providing a continuous delivery of dexamethasone directly to the site of inflammation allowing for clinical effect for up to 30 days (1).
INDICATION AND USAGE
DEXYCU® (dexamethasone intraocular suspension) 9% is indicated for the treatment of postoperative inflammation.
Please see Important Safety Information below and link to full Prescribing Information.
What types of patients are appropriate for DEXYCU?
Any cataract surgery patient who would normally receive topical steroid eye drops after surgery to treat inflammation would be appropriate for DEXYCU. It may eliminate the need for steroid drops, which in some regimens can include 70 or more drops over four weeks (4). The percentage of patients who received DEXYCU 517-mcg who had anterior chamber cell clearing on day eight was 60 percent (n =94/156) vs 20 percent (n =16/80) in the placebo group (1). The cumulative percentage of subjects receiving rescue medication of ocular steroid or nonsteroidal anti-inflammatory drug (NSAID) by day 30 was significantly lower in the DEXYCU 517-mcg treatment group (20 percent; n =31/156) compared to placebo (54 percent; n =43/80) (1).
What do the clinical trial results mean to you?
The trial showed a clinically significant efficacy in clearing of anterior chamber cells at day eight compared to placebo (1). The safety results were very compelling. Mean intraocular pressure increased minimally on day one. Mean IOP elevations in the placebo and DEXYCU treatment groups were transient at day 1 and returned to baseline by day 3 (3). The most common side effects with DEXYCU were increased intraocular pressure, corneal edema and iritis (1).
What is important to consider when administering DEXYCU?
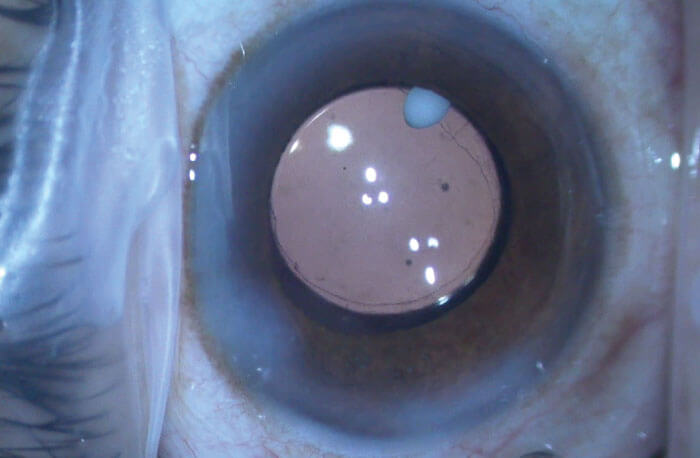
When administered according to package directions, a 0.005 ml droplet of DEXYCU containing 517-mcg of dexamethasone is placed behind the iris (1), where it forms a 2 mm spherule of liposome emulsion that adheres to the iris, capsule or even the IOL. A greater than desired dose forms a larger spherule, which, if it rests in the inferior angle instead of behind the iris, may contact the corneal endothelium, causing transient localized corneal edema. During surgery, if a droplet larger than 2 mm is observed, it may be partially aspirated from the eye to yield the correct sized dose. The most commonly reported adverse reactions in 5 percent to 15 percent of subjects included increases in IOP, corneal edema and iritis (1).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
None.
Please see continued Important Safety Information below and link to full Prescribing Information.
How has your / your patients’ experience been with DEXYCU?
I have found DEXYCU may eliminate the need for postoperative steroid eyedrops in the majority of patients. Some patients or their family will see the white droplet inside the eye if it moves from behind the iris into the inferior angle or onto the anterior surface of the iris. When educated, patients understand that this “pearl” inside the eye is releasing medication to help their healing. I still use antibiotic drops for three days pre-op and seven days post-op in all patients, and a once-a-day NSAID drop for three days pre-op and four weeks post-op in the majority of patients. Some patients may require supplemental topical steroid, especially those prone to more prolonged post-operative inflammation – for example, those with dark brown iris, diabetes, history of uveitis or patients who have had more complex cataract surgery.
Mark Pyfer is a Cataract and Refractive Specialist, Vice President and Surgical Director at Northern Ophthalmic Associates. He is also Adjunct Surgical Faculty Member at Wills Eye Hospital, Philadelphia, USA.
IMPORTANT SAFETY INFORMATION (cont’d)
WARNINGS AND PRECAUTIONS
Increase in Intraocular Pressure
• Prolonged use of corticosteroids, including DEXYCU, may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision
• Steroids should be used with caution in the presence of glaucoma
Delayed Healing
• The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation
• In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of corticosteroids
Exacerbation of Infection
• The use of DEXYCU, as with other ophthalmic corticosteroids, is not recommended in the presence of most active viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal disease of ocular structures
• Use of a corticosteroid in the treatment of patients with a history of herpes simplex requires caution and may prolong the course and may exacerbate the severity of many viral infections
• Fungal infections of the cornea are particularly prone to coincidentally develop with long-term local steroid application and must be considered in any persistent corneal ulceration where a steroid has been used or is in use. Fungal culture should be taken when appropriate
• Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection
Cataract Progression
• The use of corticosteroids in phakic individuals may promote the development of posterior subcapsular cataracts
ADVERSE REACTIONS
• The most commonly reported adverse reactions occurred in 5-15% of subjects and included increases in intraocular pressure, corneal edema and iritis
Please see full Prescribing Information for DEXYCU.
DEXYCU and the DEXYCU logo are registered trademarks and the EyePoint logo is a trademark of EyePoint Pharmaceuticals, Inc. Verisome is a registered trademark of Ramscor, Inc.
©2019 EyePoint Pharmaceuticals, Inc. All rights reserved. 480 Pleasant Street, Suite B300, Watertown, MA 02472
11/2019
US-DEX-1900163
References
- DEXYCU (dexamethasone intraocular suspension) 9% full US Prescribing Information. EyePoint Pharmaceuticals, Inc. December (2018).
- Data on file, EyePoint Pharmaceuticals, Inc.
- N Haghjou et al., “Sustained release intraocular drug delivery device for treatment of uveitis”, J Ophthalmic Vis Res, 6, 317-329 (2011).
- E Donnenfeld and E Holland, “Dexamethasone intracameral drug-delivery suspension for inflammation associated with caratact sugery: a randomized, placebo-controlled, phase III trial”, Ophthalmology, 125, 700-806 (2018).
