Non-adherence to daily topical therapies in glaucoma management poses a significant barrier to successful disease prevention and control. With non-adherence rates ranging from 30 to 80 percent, eliminating the need for self-administered medication has become a key priority for drug developers (1).
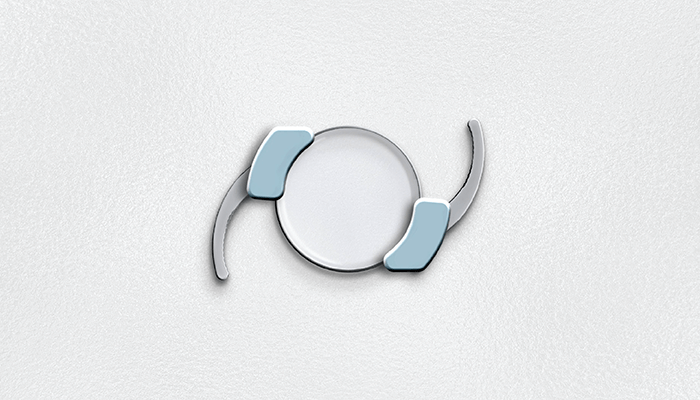
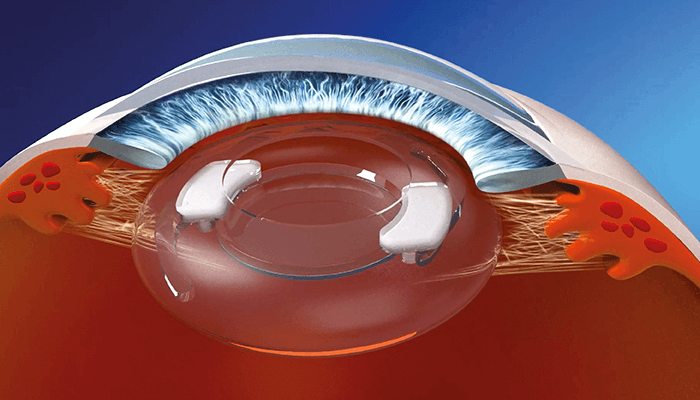
Tackling the issue of non-compliance head on, SpyGlass is a new drug delivery platform developed by Power Lister Malik Y. Kahook (2). Consisting of a hydrophobic acrylic intraocular lens (IOL) with two bimatoprost drug dispensers, the device is injected into the eye’s capsular bag at the time of cataract surgery. Once in place, SpyGlass releases consistent levels of medication for up to three years – lowering intraocular pressure (IOP), while avoiding the side effects affiliated with topical eye drops.
In light of the first human study, which showed a 45 percent reduction in IOP levels (1), we caught up with Kahook to find out more.
What inspired the development of SpyGlass – and then the spinout company SpyGlass Pharma?
SpyGlass started as a collaboration between me and Glenn Sussman. Glenn and I set out to address one of the biggest unmet needs in ophthalmic care – the widespread levels of poor adherence to therapy. We know that most patients don’t take their prescribed topical therapeutics the majority of the time for a number of reasons, including adverse events (burning, stinging, blurry vision), inability to properly aim drops onto the eye, forgetfulness, among others.
Our goal was to create a platform that can deliver medications from weeks to multiple years after implantation into the eye at the time of cataract surgery. The first disease we are targeting is glaucoma, using a prostaglandin analogue. We started the journey about four years ago and we are very happy that our vision is now a reality with the first in human study showing great efficacy.
What challenges did you encounter during development?
There are always so many challenges when developing new technologies and therapeutics! The best way to work through these issues is to be patient and build for the future. We have excellent team members with complementary skill sets which is one key to successful innovation. When issues arise, we can use the collective knowledge of the experienced team to identify the issue and implement solutions with minimal time wasted.
What is the safety profile of this treatment?
Our technology consists of a hydrophobic acrylic intraocular lens (IOL) with two bimatoprost drug dispensers that have a payload of three years. Given that hydrophobic acrylic IOL materials have been used for decades and bimatoprost has also been in use as a topical therapy for treating glaucoma for decades, the safety profile of our approach is excellent and one of the significant value propositions. Our preclinical and clinical data also support the safety profile of our technology and we look forward to continued development towards commercialization.
Why is this new treatment so exciting for the field of glaucoma?
Put simply: 100 percent adherence without any of the issues relating to topical therapy. The multi-year payload and ability to address glaucoma at the time of cataract surgery are also major advantages of our approach.
What are the next stages for this treatment?
We will be filing for our IND very soon and the whole team is looking forward to the exciting challenges of recruiting and enrolling for the phase I/II study in the coming months.
References
- University of Colorado Anschutz Medical Campus, “Drug Delivery Platform Developed by CU Ophthalmologist Shows Promise for Glaucoma Patients” (2023). Available at: https://bit.ly/3UIfVnt.
- The Ophthalmologist Power List, “Malik Y. Kahook” (2023). Available at: https://bit.ly/42FOn4Y.
