
- The most effective form of corneal collagen cross-linking (CXL) requires corneal epithelial cell abrasion, riboflavin soaking (30 minutes), constant riboflavin application during UV light exposure for an additional 30 minutes
- Decorin core protein is a naturally-occurring proteoglycan that, when applied to the cornea, could have a similar strengthening and cross-linking effect as UV-A riboflavin CXL
- Some preclinical corneal biomechanical evaluations of decorin core protein have been conducted on pig and donor human corneal pairs
- The results so far are encouraging, with decorin-treated corneas showing similar biomechanical changes to what would be expected with standard UV-A riboflavin CXL treatment
Corneal collagen cross-linking (CXL) with ultraviolet (UV)-A light and riboflavin is a relatively young procedure that has made a huge impact for the treatment of keratoconus and other corneal ectasias. UV-A light activates riboflavin, producing oxygen radicals that induce the formation of collagen cross-links, mechanically strengthening the cornea, and increasing its resistance to proteases that are upregulated in these corneal diseases.
The protocol that reproducibly gives the best results is still Wollensack and Spoerl’s Dresden protocol (1):
- Perform corneal abrasion to remove the epithelial cells to expose the collagen in the corneal stroma;
- Soak in riboflavin for 30 minutes;
- Irradiate the eye for 30 minutes of mW/cm2 of UV-A illumination;
- Meanwhile riboflavin solution is continually applied to the cornea, about every 5 minutes.
People have tried leaving the epithelium intact (epi-on) and have tried a number of methods to improve riboflavin penetration through to the corneal stroma and more intense illumination in order to increase the comfort and shorten the duration of the procedure, respectively (2), but for the moment, at least, the Dresden protocol is the one that works best for most people. But what if there was an intervention that didn’t take upwards of an hour to perform, require UV illumination or abrasive removal of corneal epithelial cells? Decorin proteoglycan (decorin) is a small, naturally occurring proteoglycan, that’s involved in a number of physiological processes in the immune system and in the vasculature. Crucially, it’s present in the cornea, where it acts to bridge natural collagen fibers, organizing and stabilizing the collagen architecture. A recombinant human form of decorin (Galacorin; Euclid Systems Corp, Herndon, VA, USA/ Catalent Pharma Solutions, Somerset, NJ, USA) has been produced and used to determine its effect on corneal biomechanics.
To do so, the investigators obtained both human donor (5 pairs) and pig corneas (4 pairs; Figure 1a). A paired study design was employed in both cases – one eye was selected at random to receive decorin treatment, the fellow eye was used as a control. The epithelium was intact in all eyes, and of the five human donor pairs, one pair was excluded because the pachymetry was more than 850 µm, leaving a total of four human donor pairs for experimentation.
The decorin cross-linking procedure requires three steps and an eyecup (Figure 1b):
- Instillation of pretreatment solution for 45–60 seconds;
- Penetration enhancer solution instillation for 45–60 seconds, followed by rinsing;
- Application of the decorin core protein for 45–60 seconds, followed by rinsing.
Including rinsing steps, the total treatment time was less than 4 minutes per eye. The pig corneas were investigated using uniaxial testing (Figure 1c) – where strips of the cornea were placed under tension with a Rheometric Systems analyzer (TA Instruments, New Castle, DE, USA), as a marker of corneal strength and elasticity, and the stress results were compared using a paired t-test.
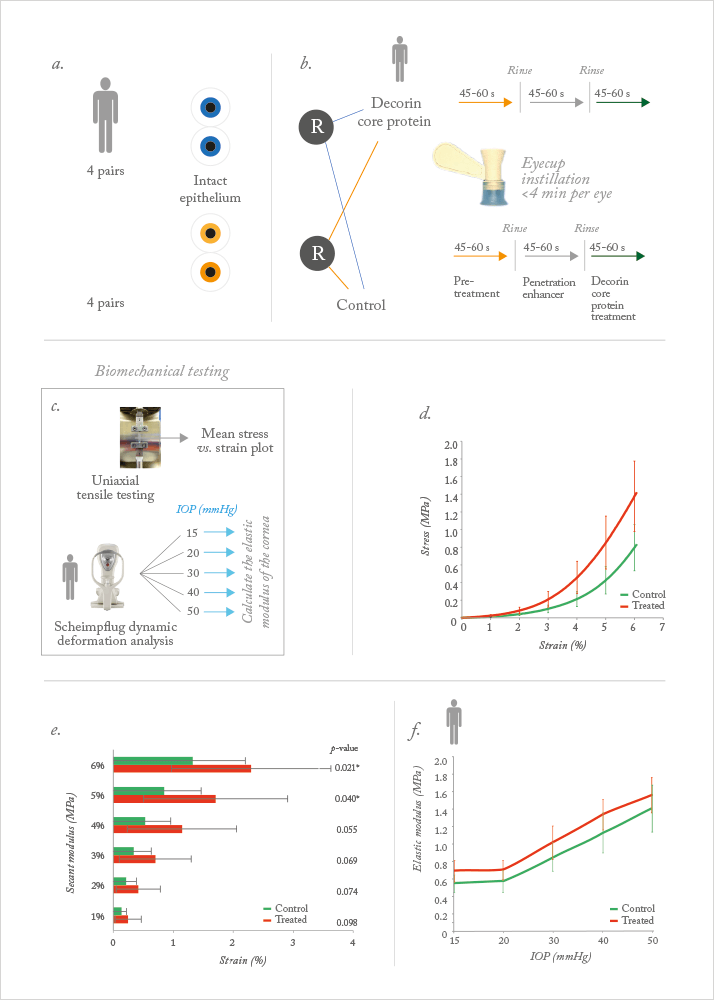
The human eyes were secured in a custom mount, then dynamic Scheimpflug deformation analyses was performed using a Corvis ST (Oculus, Wetzlar, Germany) at intraocular pressures (IOPs) of 15, 20, 30, 40 and 50 mmHg (Figure 1c). Analysis of variance (ANOVA) was performed on the deformation parameters based on the treatment and pressure effects, to determine whether there was a difference between the treated and control eye. The elastic modulus for each IOP level was calculated, using the equations of applanation (3), and parameters derived from the dynamic Scheimpflug images. Treated pig eyes were significantly stiffer than the untreated eyes, specifically at 5 and 6 percent strain (Figure 1d). In reviewing the secant modulus at 6 percent strain, the investigators observed a 73.2 percent increase in the stress value (Figure 1e) – which is very similar to that recorded by Wollensack, Spoerl and Seiler (4) when they performed stress-strain measurements of UV-A/riboflavin on pig corneas (71.9 percent). The difference at 4 percent strain approached significance, but is likely a reflection of the low number of animals used – four.
But what would be seen with the dynamic Scheimpflug deformation analyses in human corneas? Stiffer tissue in this case would have greater resistance to deformation, leading to lower deformation amplitude and slower movement, much like what would be expected after traditional CXL. Furthermore, UV-A riboflavin CXL also results in thinner and flatter corneas. Would this be observed?
The result was that decorin treatment of the cornea had statistically significant effects on pachymetry, initial radius of curvature, maximum deformation amplitude, A1 length, A1 velocity, A1 deformation amplitude, as well as the modulus of elasticity – in other words, decorin treatment produced changes in the cornea that were consistent with both stiffening and cross-linking (Table 1; Figure 2). IOP had a statistically significant effect in many of the parameters – all except one or two – and there were no significant interaction terms (Figure 1f).
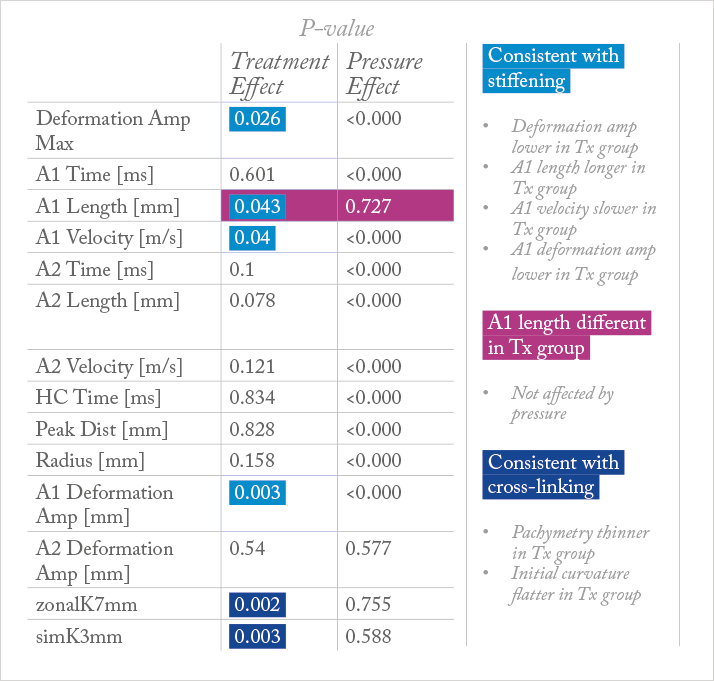
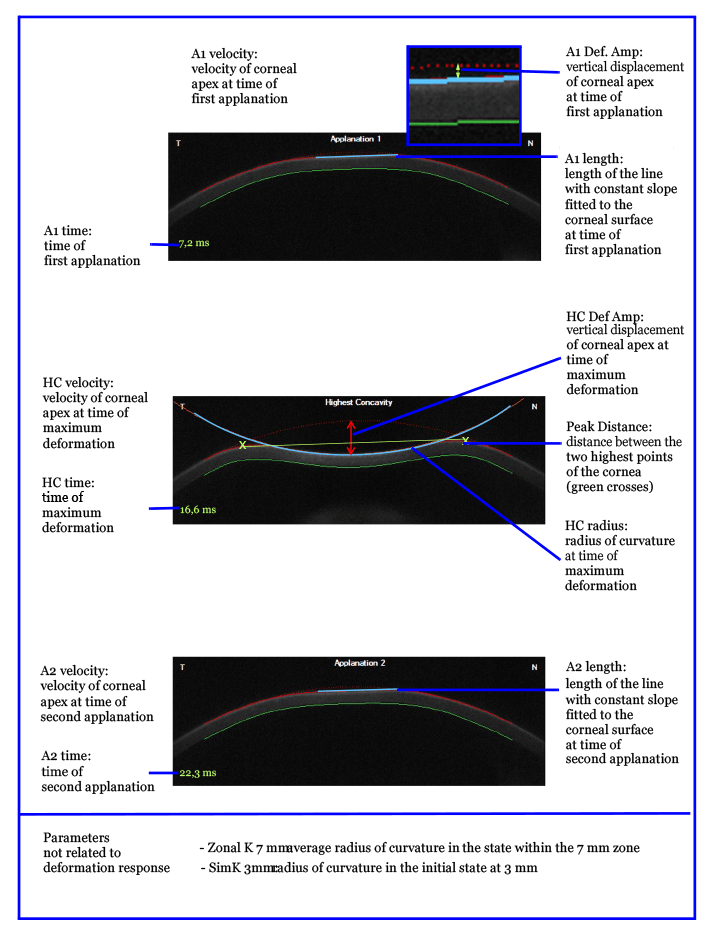
In one of the pairs, the Scheimpflug deformation analysis videos at 15 mmHg IOP were pseudocolored and overlapped – the decorin-treated eye was colored blue, and the untreated one, colored yellow (see Video). The video shows that there is a significant stiffening behavior in the deformation of the decorin-treated cornea relative to the untreated fellow eye. The modulus of elasticity data revealed a significant treatment effect and effect of IOP, but no interaction effect (Figure 1f).
The researchers concluded that treatment with decorin core protein appears to produce a higher elastic modulus and stiffer biomechanical behavior in both human and porcine corneas. They plan to add greater numbers in the future to confirm this with a larger sample size, but what we have here is a protein that can be applied to the cornea, in a procedure that lasts under four minutes, without the requirement for UV light, that in preclinical models, can strengthen the cornea in a similar manner to UV-A riboflavin CXL. Further study is required, but this could prove to be a promising – and epi-on – alternative to UV-A riboflavin CXL for patients with corneal ectasias like keratoconus, and forthe stabilization of corneal structure following LASIK surgery.
From a Presentation at the 10th International Congress of Corneal Cross-Linking in Zurich, Switzerland, December 6, 2014 by Cynthia J. Roberts, Kimberly M. Metzler, Ashraf Mahmoud and Jun Liu. Cynthia J. Roberts is Professor of Ophthalmology and Biomedical Engineering and the Martha G. and Milton Staub Chair for Research in Ophthalmology at The Ohio State University. Kimberly M. Metzler, is a recent graduate in Biomechanical Engineering from The Ohio State University in Biomedical Engineering. Ashraf M. Mahmoud, is a Systems Developer in Ophthalmology and Biomedical Engineering at The Ohio State University. Jun Liu, is Associate Professor of Biomedical Engineering and Ophthalmology at The Ohio State University.
References
- G Wollensak, et al., “Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus”, Am J Ophthalmol, 135, 620–627 (2003). PMID: 12719068. F Raiskup, et al., “Epi-on or Epi-off?”, The Ophthalmologist, 17, 28–33 (2015). bit.ly/Raiskup J Liu, CJ Roberts, “Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis”, J Cataract Refract Surg, 31, 146-155 (2005). PMID: 15721707. G Wollensak, et al., “Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking”, J Cataract Refract Surg, 29, 1780–1785 (2003). PMID: 14522301.
