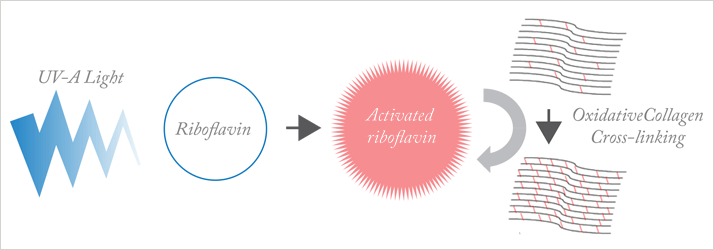
 Figure 1. Main principles of photo-oxidative corneal cross-linking. Ultraviolet-A (UV-A) light activates riboflavin applied to the cornea; activated riboflavin causes oxidative cross-linking of corneal collagen, stabilizing the corneal structure.
Figure 1. Main principles of photo-oxidative corneal cross-linking. Ultraviolet-A (UV-A) light activates riboflavin applied to the cornea; activated riboflavin causes oxidative cross-linking of corneal collagen, stabilizing the corneal structure.
CXL stabilizes keratoconus, and can improve it
Optimal combinations of CXL with other treatments are not fully defined
Development of the technique is on-going
Implementation in the US lags behind Europe
Picture this scenario: 25-year old quadruplets, each one living in a different European country, develop keratoconus at the same time. Keratoconus is a degenerative condition characterized by conical protrusion of the cornea and irregular astigmatism. The four siblings each present to their local expert ophthalmologist who specializes in surgical management; all four want their vision improved rather than just stabilized. Six months after treatment is completed, all four quadruplets are satisfied that their vision has improved, and the keratoconus has been stabilized in all cases. Yet the four identical cases were managed quite differently. The first quadruplet was offered topography-guided photorefractive keratectomy with corneal cross-linking (CXL) to improve and stabilize vision. The second received intracorneal rings followed by CXL some months later. The third was treated with CXL and a phakic intraocular lens implant (IOL) to correct the residual refraction following the CXL procedure. And the fourth had a thermal procedure – either microwave thermoplasty (Keraflex) or conductive keratoplasty (CK) – followed by CXL, to improve vision and stabilize the keratoconus. The only common component of treatment is CXL.
There genuinely is this much disparity in ‘best practice’ across the continent. It’s a function of the regulatory climate in which a device can be used to treat patients once it has been awarded a CE mark, which denotes compliance with EU product legislation. This contrasts with the US, where ophthalmologic technology must undergo clinical trials and demonstrate benefit before receiving approval for a specific indication (and where CXL is not yet in routine practice since sponsorship issues led to trials being halted, so FDA approval is still awaited). Are the various approaches used in Europe equally good – a version of “the lottery where everyone wins” seen on The Simpsons television show – or is there truly a best option? It’s not an easy question to answer. Ideally, in my view, new devices would undergo randomized, controlled clinical trials in Europe to generate a safety and efficacy evidence base before widespread adoption.
Exploring CXL
Despite the issues mentioned above, the utility of CXL, a technique which uses UV-A light and a photosensitizer to strengthen chemical bonds in the cornea is not in doubt (see ‘CXL Theory and Practice’). My practice introduced the approach in January 2007, following EU-approval of the first illumination lamp. Before then, only a small number of European centers performed CXL as part of clinical trial programs for keratoconus and post-laser-assisted in-situ keratomileusis (LASIK) ectasia. Today, while CXL might still be considered to be a new procedure, there is ample published support for its effectiveness. In many CXL-treated eyes, not only is the rate of keratoconus progression slowed or even stopped, but some of the corneal steepening is actually reversed (Figs. 3 & 4). Patient expectations must be managed to reflect the fact that only around 70 percent of those who undergo the technique experience improved vision. The primary use of CXL is in cases where the keratoconus and its associated corneal steepening and astigmatism continue to progress despite treatment with a rigid gas-permeable contact lens (RGP-CL). Until January 2011, the two main recommendations for CXL were in cases where there is evidence of keratoconus progression and where an RGP-CL-wearing patient finds that they can no longer wear the lens and faces keratoplasty (either lamellar or full-thickness). These recommendations were modified following the 7th CXL Meeting held in Milan, Italy in January 2011, where it was recognized that the chances of keratoconus disease progression is high; it was agreed that patients with keratoconus aged 27 years or younger should undergo CXL following diagnosis, rather than waiting for evidence of disease progression. CXL is not without its share of complications. The first step is to remove the epithelium; this will cause pain or discomfort, and can take three to five days to heal. Complications can occur, including infection, delayed healing, scarring and loss of best corrected visual acuity (BCVA). These have led to the practice of performing CXL without corneal epithelium removal, using a procedure known as “epi-ON” CXL. Epi-ON CXL is less painful, safer, and results in a more rapid return to vision and work in general. However, questions remain regarding the effectiveness of epi-ON CXL. Studies have shown it to be less effective than epi-OFF, and it usually provides little or no overall benefit to the patient (1). Newer approaches to epi-ON CXL are currently being studied, including iontophoresis – also known as electromotive drug administration – and other technologies to increase the amount of riboflavin (the key substrate in CXL reaction) in the corneal tissue. Initial data looks promising, but longer term follow-up is required.
CXL involves a single application of a photosensitizer – riboflavin (vitamin B2) – to the eye, followed by 30 minutes of UV-A light (Fig. 1) to activate the riboflavin. Activated riboflavin causes new bonds to form across adjacent collagen strands in the stromal layer of the cornea (Fig. 2); this cross-linking helps to recover and improve the mechanical strength of the cornea, stabilizing and potentially improving keratoconus.
CXL Theory and Practice
CXL involves a single application of a photosensitizer – riboflavin (vitamin B2) – to the eye, followed by 30 minutes of UV-A light (Fig. 1) to activate the riboflavin. Activated riboflavin causes new bonds to form across adjacent collagen strands in the stromal layer of the cornea (Fig. 2); this cross-linking helps to recover and improve the mechanical strength of the cornea, stabilizing and potentially improving keratoconus.
 Figure 2. Patient undergoing the corneal cross-linking procedure with the IROC UV-X 2000.
Figure 2. Patient undergoing the corneal cross-linking procedure with the IROC UV-X 2000.
An expert group, under the chairmanship of Jerome Vryghem, will meet in October 2013 prior to the ESCRS conference in Amsterdam to discuss best means of determining the success and failure of different CXL procedures. These are fundamental issues that still need to be addressed. Most ophthalmologists use the decreases in average and maximum keratometric values (K-max, see Fig. 3) as parameters to follow pre- and post-operatively as a measure of the success or failure of CXL. Alas, in reality these parameters may not be specific or robust enough to accurately reflect the efficacy of the procedure. It has been suggested by A. John Kanellopoulos that surface variation and height decentration indices (ISV and IHD) are parameters that correlate more closely with the patient’s subjective reports on their vision. Currently, the American-European College of Ophthalmic Surgery (ACOS) and Avedro are performing a CXL safety and efficacy study involving up to 100 US-based study sites for the treatment of keratoconus. More than 100 studies have already been published addressing the safety and efficacy of CXL for keratoconus and post-LASIK ectasia. Most employ the initial Dresden protocol, that is, epithelial removal, riboflavin loading for 25–30 minutes, followed by 3 mW ultraviolet-A (UV-A) light for 30 minutes (2-3). Some studies have employed higher-intensity illumination for shorter periods (4) including one, dubbed AXL – the accelerated CXL study – at my own institute. This uses the IROC UV-X 2000, which has an optimized beam profile that compensates for the peripheral part of the cornea where efficacy is typically lost due to corneal curvature and increased corneal thickness in the periphery. Ten minute treatment exposes the central cornea to the same total energy that the Dresden protocol provides in 30 minutes. This study has concluded enrolment. Follow-up data at one-year is not yet available but at the moment it appears that the AXL protocol causes more corneal flattening than the 3mW lamp, albeit potentially with increased corneal inflammation. Studies performed at University College Dublin’s Applied Optics Department demonstrated that a beam intensity of >30mW had limited CXL effect on porcine corneas imaged with second-harmonic imaging; 3 mW and 10 mW treatments appeared to have similar effects (5). Speaking to fellow ophthalmologists who have access to different CXL systems, there appears to be some agreement that the ideal illumination time is probably around the 10 to 15 minute mark when using a lamp that can deliver the same total energy as the Dresden protocol.
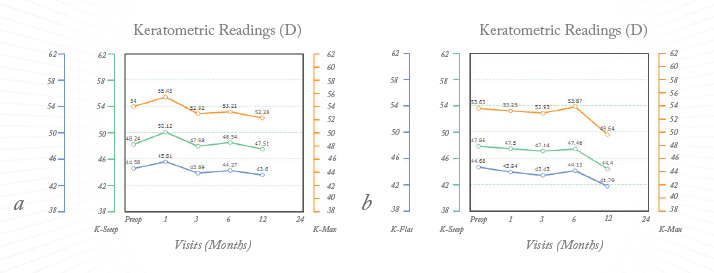 Figure 3. Corneal curvatures after corneal cross-linking (A) and accelerated corneal cross-linking (B). The orange line represents K-Max while the green and blue lines represent the central 3mm steep and flat Ks respectively.
Figure 3. Corneal curvatures after corneal cross-linking (A) and accelerated corneal cross-linking (B). The orange line represents K-Max while the green and blue lines represent the central 3mm steep and flat Ks respectively.
Combination treatments
Returning to our quadruplets, one of the procedures used, topography-guided PRK, has over nine years of follow-up data (6). I have six years of follow-up using a variant of this treatment, called “SimLC” – simultaneous laser CXL – that specifically only treats the topography component on the laser ablation (PRK) with no refractive component. Other procedures (such as the Athens protocol) treat some of the refraction depending on the ablation depth and the existing corneal thickness. With SimLC we never ablate to a greater depth than 50 µm and therefore do not address any refractive errors. Topography-guided PRK always flattens the cone and steepens the superior flat cornea, through the use of hemi-hyperopic treatment at the superior far periphery of the cornea. SimLC typically flattens K-Max by 5.9 D on average, whereas in our experience regular CXL flattens K-Max by 2.1 D – in the 70 percent of cases where some flattening does occur. A quarter of my patients receive SimLC, and have the qualifying factors: enough corneal tissue (>470 µm), reduced BCVA (normally worse than 6/10) and a specific desire to want to improve their quality of vision. Intracorneal rings are also well represented in the literature, and their use will often improve corneal astigmatism as well as improve myopic refraction. To be suitable for intracorneal rings, the corneas need to be sufficiently thick at the zone where the channel is to be created – either mechanically or using the femtosecond laser. Implantable collamer lenses (ICLs) – whether toric or non-toric – have also been widely used to correct the refractive errors that exist after CXL has stabilized the cornea. The IOL can be placed as soon as the corneal shape changes have stabilized, often around the six to 12 month post-treatment mark. It is also possible to carry out CXL of the cornea when the eye already has a phakic IOL in situ. The thermal procedures have the advantage that, unlike the PRK procedure, they do not remove tissue nor, unlike the intrastromal ring procedures, do they implant material into the cornea. However, they are unstable when used alone – the thermal procedures certainly bring about positive corneal shape changes, but in the absence of any CXL the corneal shape regresses to pre-operative levels within about three months CXL applied shortly after the patient undergoes the thermal procedure greatly enhances the longevity of the thermal procedure’s effect (7). A presentation at the AAO in October 2012 showed that the timing of CXL relative to the thermal procedure was important (8). Simultaneous CXL failed to add stability to the outcomes, but CXL carried out at six hours or later did. The most successful cases already have two-year follow-up and are still demonstrating beneficial effects of the thermal procedure.
If you encounter four surgeons, who each provide just one of the procedures above, they will typically be passionate about their protocol and defend it strongly. Those surgeons who provide two, three or even all four of the procedures will use a decision tree to determine which is the most suitable for the patient. Nevertheless, a consensus will only be reached once formal clinical trials have been conducted, and the outcomes analyzed. Until then, surgeons will perform the procedures that they believe in and will continue to believe that they have provided their patients with the best possible outcomes.
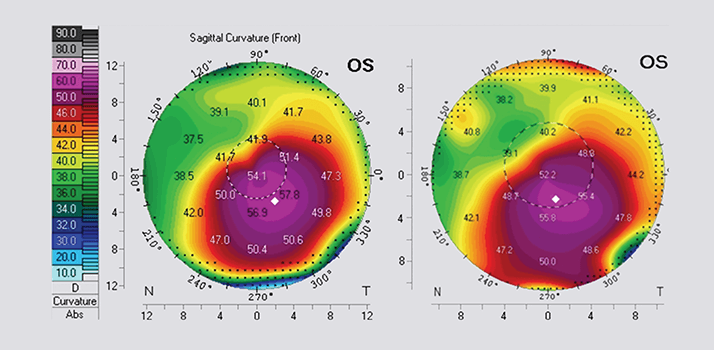 Figure 4. Pre-operative Pentacam image (A) and 1 year post- corneal cross-linking image (B). The central Ks have flattened from 54.1 to 52.2D and the steepest Ks from 57.8 to 55.8D.
Figure 4. Pre-operative Pentacam image (A) and 1 year post- corneal cross-linking image (B). The central Ks have flattened from 54.1 to 52.2D and the steepest Ks from 57.8 to 55.8D.
LASIK Xtra
A CXL technique that is starting to make inroads into clinical practice is LASIK Xtra. This technique involves a short burst of CXL – termed “flash CXL” – that is applied to the LASIK bed after the laser ablation has been completed and the anterior stroma has been soaked with riboflavin for a shorter than usual period: the normal soak time is around 20 minutes, but with LASIK Xtra this is only one to two minutes. The illumination phase period duration is also greatly reduced – normally to half of what the standard would be with that particular lamp. LASIK Xtra is used to treat high myopia when the residual corneal thickness is less than 300 µm – especially in young women who may become pregnant in the future (9). Some ophthalmic surgeons have also adopted LASIK Xtra procedures for hyperopia, as it has been shown that it stabilizes the refractive and corneal topography changes induced by hyperopic LASIK. The procedure is particularly appealing from the patient’s perspective, because LASIK is known to weaken the cornea by 10 to 30 percent (depending on the flap thickness and the ablation depth) and applying CXL strengthens the corneal fibers and increases rigidity. If CXL is applied in an abbreviated, flash form at the end of the LASIK procedure, the patient can leave the operating room with improved vision and a cornea that is potentially no weaker than it was prior to surgery. Further studies are required to determine efficacy, safety, techniques and dosages to ensure optimal treatment, but this does appear to be fulfilling much of its initial promise.
Conclusion
CXL is a vital and innovative tool. By stabilizing progressive keratoconus, it will reduce the number of corneal transplants required. It has been combined with other procedures to improve the vision in patients with keratoconus, and has made it easier to wear rigid gas permeable and soft toric contact lenses. Most significantly, it halts progressive corneal steepening for LASIK ectasia cases. Further studies of CXL are needed, particularly to inform guidelines to help physicians decide where, when, and what procedures are best suited to individual patients. We may soon view today’s techniques as crude, but CXL is a positive (for the most part) contribution to improving the lives of patients with keratoconus. Arthur Cummings is a Consultant Ophthalmologist at the Wellington Eye Clinic and UPMC Beacon Hospital, Dublin, Ireland.
Videos
Indications for CXL:
Topography-guided PRK and CXL for Keratoconus:
http://eyetube.net/series/acos-dulaney-winter-meeting-2012-daily-coverage/topo-guided-prk-and-cxl-for-keratoconus--noosq/ Keraflex:
http://eyetube.net/video/keraflex-procedure/ LASIK Xtra:
http://eyetube.net/series/eyetube-tv-show-daily-vienna-2011/crosslinking-after-laser-vision-correction-in-normal-eyes/; http://eyetube.net/series/2013-acos-ophthalmic-solutions-in-cataract-refractive-surgery/lasik-and-cxl--sideg/
References
- Wollensak G, Iomdina E. Biomechanical and histological changes after corneal cross-linking with and without epithelial debridement. J Cataract Refract Surg. 2009; 35:540–546. Wollensak G, Spoerl E, Seiler T. Riboflavin/ ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135:620–627. Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety Of UVA riboflavin cross-linking of the cornea. Cornea. 2007; 26: 385–389. Schumacher S, Oeftiger L, Mrochen M. Equivalence of Biomechanical changes Induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011; 52: 9048–9052. Cummings AB, McQuaid R. Accelerated CXL versus Standard CXL: 2nd Harmonic Imaging of Porcine Corneas. Paper. CXL Congress 2012. Kanellopoulos AJ, Binder PS. Collagen Cross-Linking (CCL) With Sequential Topography- Guided PRK: A Temporizing Alternative for Keratoconus to Penetrating Keratoplasty. Cornea. 2007; 26: 891–895. Nguyen MK, Chuck RS. Corneal collagen cross-linking in the stabilization of PRK, LASIK, thermal keratoplasty, and orthokeratology. Curr Opin Ophthalmol. 2013;24:291–295. Cummings AB. Factors Influencing the Stability of Keraflex Treatments for Keratoconus. Optom Vis Sci 2012;89:E-abstract 125080. Hafezi F, Koller T, Derhartunian V, Seiler T. Pregnancy may trigger late onset of keratectasia after LASIK. Letter to Editor. J Refract Surg. 2012; 28 (4): 242–243.
