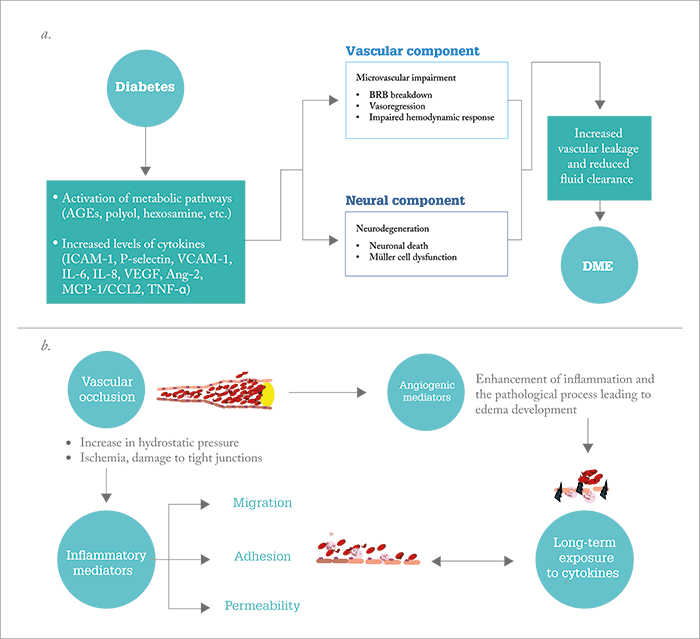
- There’s a good reason that steroids are implicated in the treatment of DME and RVO – the pathogenesis of both these diseases is driven also by inflammation
- Despite the fact that steroids can suppress the expression of multiple inflammatory mediators, many ophthalmologists turn to anti-VEGFs first, which carry a high treatment burden
- It’s important to remember that steroids may be a suitable option for many patients
- Here, I review intraocular steroid treatments as an option for DME and RVO, covering both key clinical research, and real-life outcomes
When treating retinal disease, most ophthalmologists will turn to anti-VEGFs first, and for many patients who are phakic and can attend frequent follow-ups, this is a reasonable approach. But there are many patients in whom it actually makes sense to use steroids instead, and in this article I will outline why.
A tale of two diseases
The main diseases where we use intraocular steroids are diabetic macular edema (DME) and retinal vein occlusion (RVO). Although each has distinct pathogenic mechanisms, one common element unites them both: the presence of inflammation and elevated cytokines (see Figure 1). In DME, there are increased levels of multiple disease-promoting cytokines that correlate with the severity of disease (1)(2)(3), and which steroids are known to block. In a study by Sohn et al., steroids were shown to address the multifactorial nature of DME by targeting both inflammatory mediators and VEGF (4): in patients with DME who were bilaterally injected with 1.25 mg bevacizumab and 4 mg triamcinolone acetonide (TA), levels of VEGF decreased in the bevacizumab-injected eyes as expected (p<0.01), but in the TA-injected eyes, levels of both disease-promoting cytokines and VEGF were decreased significantly (p<0.016 and p=0.050, respectively) (4). Clearly, from a mechanistic perspective, using steroids in DME makes sense.In RVO, inflammation also plays a big role in disease progression. Tellingly, IL-6 is a better discriminator of the condition than VEGF, because vitreous IL-6 levels in patients with central RVO (CRVO) are significantly different from the levels found in patients without the disease, whereas VEGF levels overlap (5). Further, hyper-reflective dots, seen on spectral domain OCT scans of patients with early RVO (which are thought to represent microglia or macrophages part of the time and potentially be an early marker of the inflammatory process in vein occlusion) disappear after treatment with dexamethasone (6). So when we consider the pathogenic components of DME and RVO it makes sense that the treatment regimen features an agent that combats all of these inflammatory triggers. The question is: what role can steroids play in the treatment of these diseases?
The evidence – DME
Let’s take a look at the evidence starting with TA first. In the Diabetic Retinopathy Clinical Research (DRCR) network Protocol I (7), TA and prompt laser treatment was compared with ranibizumab and prompt or deferred laser treatment in patients with DME.Initially, the TA arm performed much better than the sham injection arm in terms of visual acuity (VA) gains, but after one year, VA had declined to below baseline levels. This was due to cataract development, because the subset of eyes that were pseudophakic at baseline performed as well as the ranibizumab arm. Indeed, we cannot discuss steroids without mentioning side effects, and with TA, there were significant intraocular complications – IOP increased by 10 mmHg or more in almost half of the patients and nearly a third of patients required the initiation of topical IOP-lowering medication (7). So although TA showed superiority over laser treatment alone, there were also frequent complications. When Protocol I was published, TA was being investigated as a slow-release implant – but this was soon abandoned at the Phase II stage (8). Is TA the best drug for DME? A Phase I/II trial of a TA formulation in this indication (HULK) is currently underway (9), and it will be interesting to see the results.More success has been shown with the fluocinolone acetonide slow-release implant, Iluvien (Alimera), which was the first steroid to receive approval for the treatment of DME. Pharmacodynamic studies have shown excellent sustained intraocular release of the steroid, and many patients have benefitted from its long duration of action of up to three years (see Figure 2). The Phase III FAME trials (Figure 3a-c)(10), (11) were instrumental in demonstrating Iluvien’s efficacy for the treatment of DME, but it was a pre-planned sub-analysis that revealed its efficacy was actually greatest in patients who had chronic disease at baseline – defined as three years duration in one analysis, and 1.7 years in another. Since its approval, interim analysis of the real-world Iluvien Registry Safety Study (12) has shown that 60 percent of patients with chronic DME (mean duration 4.6 years) experienced VA improvements after six and 12 months (Figure 3d)(12). However, Iluvien therapy is also associated with side effects. In the FAME trial, almost 82 percent of patients developed cataract and many experienced raised IOP – with around five percent needing filtration surgery to correct it (Figure 3c)(13). Similarly, at the interim analysis of the ongoing real-world study, 18.4 percent of patients needed IOP-lowering therapy and 9.8 percent developed cataract (12).
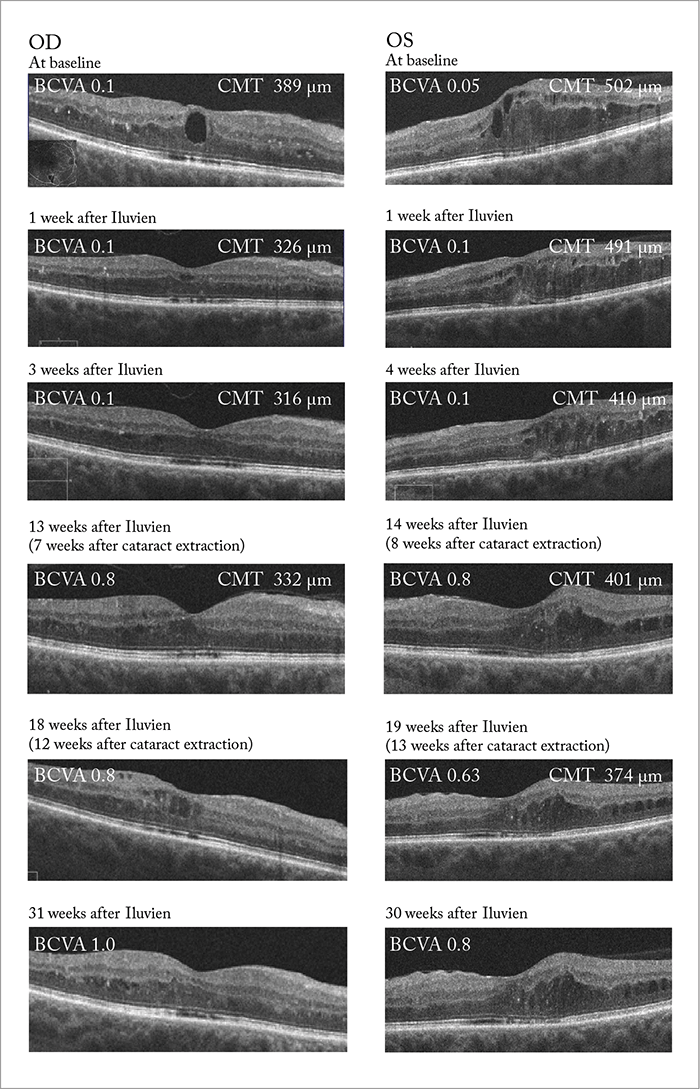
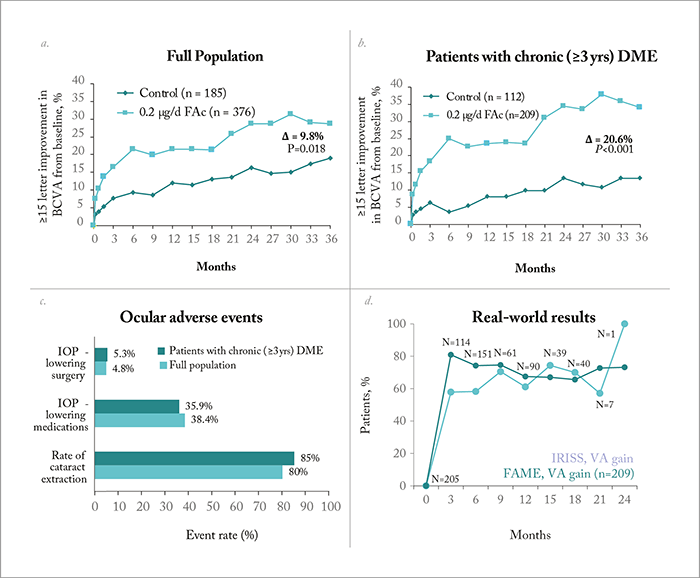
But how important are the side effects to the final outcome? It turns out not very – when we compare pseudophakic patients with patients who were phakic at baseline (and later underwent cataract surgery), they exhibit almost identical VA results. In other words, final VA is unaffected by cataract formation, so long as these patients go on to have cataract surgery. Furthermore, some patients in the control group experienced decreased vision following cataract surgery, but this didn’t occur in Iluvien-treated patients, likely because the steroid protected against post-surgical edema. It’s a similar situation with IOP increases: many patients exhibited it, but none progressed to glaucoma – and even if intervention was required to lower IOP, it did not impact on the proportion of patients that achieved a good VA at final outcome (10), (11).
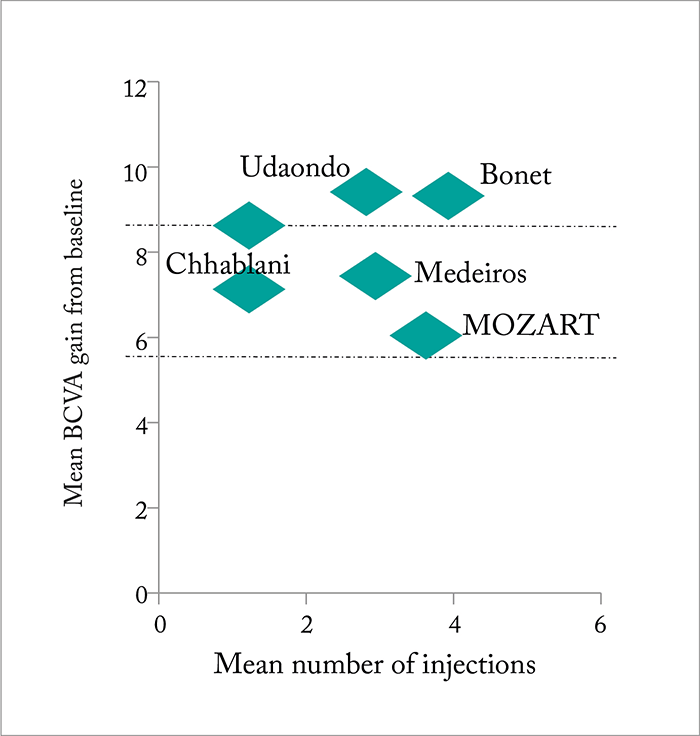
The other steroid therapy approved for the treatment of DME is the slow-release injectable implant, Ozurdex (Allergan), which has been shown to release dexamethasone over a period of around 3–4 months. The regulatory approval of Ozurdex was based on results from the PLACID, CHAMPLAIN and MEAD trials (14)(16), and of these, the two pooled Phase III MEAD trials are particularly important as they showed that significantly more patients achieved a best-corrected VA (BCVA) gain of ≥15 letters with Ozurdex compared with sham treatment (16). As with Iluvien, phakic patients experienced reductions in their VA after one year thanks to steroid-induced cataract, but consistent advantages over the sham treatment groups were seen at each timepoint in pseudophakic patients treated with Ozurdex – and cataract development (which occurred in over 60 percent of Ozurdex-treated patients) did not affect the final VA results (16). Multiple subgroup analyses of the MEAD trial have since shown that Ozurdex is effective in patients previously treated with anti-VEGF agents (17), and a number of real-life studies have reported its effectiveness in both naïve patients and those with short duration DME (see Figure 4). A key finding with Ozurdex is that eyes continue to improve with treatment – sustained improvements in retinal structure have been observed over three years of six-monthly treatment (23). The time to onset of two-step progression in diabetic retinopathy severity was also delayed by ~12 months with the 0.7 mg implant (23). The IOP increase effect also improves over time: although it’s true that around 30 percent of Ozurdex-treated eyes in the MEAD trials had an IOP increase of ≥10 mmHg – compared with 4 percent in the sham-treated eyes – IOP decreased between retreatments, and the percentage of patients with elevated IOP decreased over time (24).
This brings me to my next point: frequency of dosing. A six-monthly dosing schedule was employed in the MEAD trials, but what we know from real-life usage of Ozurdex, is that patients show better outcomes with pro re nata (PRN) dosing. This was confirmed by a recent study performed in Italy that showed a mean BCVA improvement in the PRN group of 0.14 versus 0.03 LogMAR in the group treated with the standard fixed six month regimen (25). Similarly, real-life experiences of using Ozurdex to treat RVO show improvements beyond what was seen in the pivotal trials, and this may be due to patients receiving more frequent dosing. Together, the outcomes suggest that patients should be monitored after three months, and if not treated then, thereafter monthly to see whether edema is recurring and they need retreatment.
The evidence – RVO
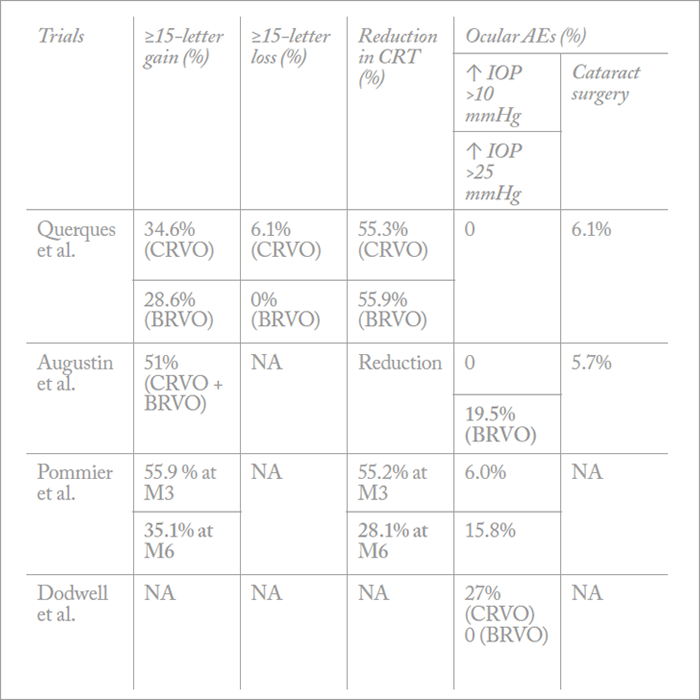
Ozurdex is also indicated for the treatment of RVO (in Europe) and the treatment of ME secondary to branched RVO (BRVO) or CRVO (in the US) based on results from the GENEVA trial (26). But although patients treated with Ozurdex in the trial (0.35 mg or 0.7 mg dexamethasone implant) showed significant improvements over the sham arm, they were under-treated. As re-treatment was only allowed every six months, the VA results simply don’t reflect what we see in real-life. Furthermore, as patients only received two injections in the GENEVA trial, the safety profile was incredibly good – less than 10 percent of patients developed cataract in the trial compared with over 50 percent in long-term follow-up studies (27). Despite patients being under-treated, we discovered several key benefits of steroids from the GENEVA trial. We know that visual improvements are rapid (statistically significant improvements were seen as early as seven days after injection), and that the peak treatment effect is at around two months. We also know from post-hoc analyses that patients with shorter durations of edema (≤90 days) are more likely to achieve better BCVA outcomes than those with longer durations (>90 days), meaning that Ozurdex should be very effective at treating early onset cases of ME (26). Follow-up results showing that the presence of active retinal neovascularization was only increased in sham-treated patients also suggest that Ozurdex might be having an effect on preventing neovascularization. Since the GENEVA trial, a great deal of real-world experience has been collected for Ozurdex in RVO (Figure 5), and we have guidelines and a consensus document showing that more than 40 percent of patients with CRVO or BRVO achieve a visual improvement of ≥15 letters after two injections (28). Other trials across Europe have shown the same results (Table 1), and one study in the US has shown a ≥3 line improvement in 50 percent of patients at six months (33). The results of these real-life trials formed the basis for the indication being changed in some European countries so that the treatment could be re-administered after four months, rather than six.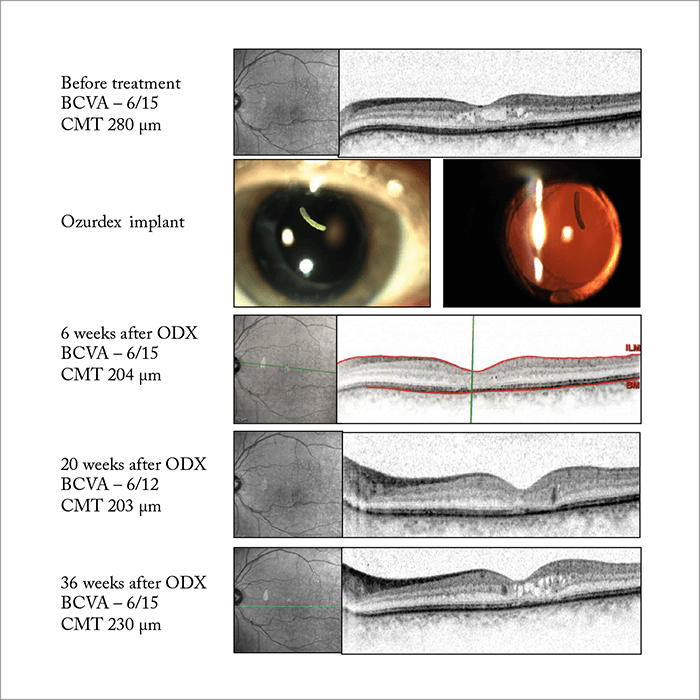
Why wait?
It should be remembered that the results of anti-VEGFs in real-life fall short in many instances because of the high treatment burden – often, patients just aren’t being treated as often as they should be. Additionally, up to 50 percent of patients receiving anti-VEGFs as first-line therapy do not respond sufficiently (34), some of them respond poorly initially or experience declining efficacy over time (35). With steroids, fewer injections are needed, and real-life outcomes have been shown to surpass those seen in clinical trials. Many may be discouraged by the ocular side effects associated with steroids, but these are easily controllable and don’t affect the final outcome of treatment. Regarding systemic (namely, thromboembolic) side effects of anti-VEGFs, the data is inconsistent, so if you have a patient who has just had a stroke or an MI, steroids should be considered as first-line treatment in these cases. It’s also more reasonable to consider first-line treatment with a steroid if a patient cannot return for frequent therapy or monitoring.
At the moment we may lack evidence confirming patient suitability for particular treatments, but there is currently a lot of effort being applied into finding phenotypes defining who may be more suited to steroids or anti-VEGFs. In the meantime, it’s important that we don’t forget that anti-VEGFs are not the only game in town to treat these diseases – steroids have much to offer. Anat Loewenstein is Professor and Director of the Department of Ophthalmology at Tel Aviv Medical Center, Sidney A. Fox Chair in Ophthalmology, and Vice Dean of the Sackler Faculty of Medicine at Tel Aviv University, Tel Aviv, Israel.
References
- S Rangamasay et al., Middle East Afr J Ophthalmol, 19, 52–59 (2012). PMID: 22346115. HJ Sohn et al., Am J Ophthalmol, 152, 686–694 (2011). PMID: 21782151. N Dong et al., “Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy”, Mol Vis, 19, 1734–1746 (2013). PMID: 23922491. HJ Sohn et al., Am J Ophthalmol, 152, 686–694 (2011). PMID: 21782151. H Noma et al., Ophthalmol, 116, 87–93 (2009). PMID: 19118700. G Coscas et al., Ophthalmologica, 226, 4–28 (2011). PMID: 21577038. The Diabetic Retinopathy Clinical Research Network., Ophthalmol, 117, 1064–1077 (2010). PMID: 20427088. ClinicalTrials.gov. Available at: http://bit.ly/MK0104. Accessed November 27, 2016. ClinicalTrials.gov. Available at: http://bit.ly/HULKDME. Accessed November 27, 2016. PA Campochiaro et al., Ophthalmol, 118, 626–635 (2011). PMID: 21459216. PA Campochiaro et al., Ophthalmol, 119, 2125–2132 (2012). PMID: 22727177. S Taylor et al., Poster presented at the Royal College of Ophthalmologists Annual Congress; May 24–26, 2016; Birmingham, England. Poster #110. ILUVIEN summary of product characteristics. Available at: http://www.medicines.org.uk/emc/medicine/27636. Accessed November 22, 2016. DG Callanan et al., Ophthalmol, 120, 1843–1850 (2013). PMID: 23706947. DS Boyer et al., Retina, 31, 915–923 (2011). PMID: 21487341. DS Boyer et al., Ophthalmol, 121, 1904–1914 (2014). PMID: 24907062. AJ Augustin et al., Br J Ophthalmol, 30, 15, 150 (2015). PMID: 26519345. P Udaondo et al., Presented at the American Academy of Ophthalmology (AAO) annual meeting; November 16–19, 2013; New Orleans, USA. Poster PO213. MF Bonet et al., Presented at the 5th World Congress on Controversies in Ophthalmology (COPHy); March 20–23, 2014; Lisbon, Portugal. Poster 20, Group A. MD Medeiros et al., Ophthalmologica, 231, 141–146 (2014). PMID: 24356099. S Guigou et al., J Fr Ophtalmol, 37, 480–485 (2014). PMID: 24813119. J Chhablani et al., Eye (Lond), 30, 426–430 (2-16). PMID: 26611849. RP Danis et al., Br J Ophthalmol, 100, 796–801 (2016). PMID: 26581718. RK Maturi et al., Retina, 36, 1143–1152. PMID: 26871523. P Lanzetta et al., Poster presented at the Association for Research in Vision and Ophthalmology (ARVO) Annual meeting; May 1–5, 2016; Seattle, USA. Poster C0055. JA Haller et al., Ophthalmol, 117, 1134–1146 (2010). PMID: 20417567. E Moisseiev et al., Eye (Lond), 27, 65–71 (2012). PMID: 23154502. G Coscas et al., Eur J Ophthalmol, 24, 1–9 (2014). PMID: 24249150. L Querques et al., Ophthalmologica, 229, 21–25 (2013). PMID: 23006995. A Augustin et al., Acta Ophthalmologica, 90, s249 (2012). S Pommier and F Meyer. Acta Ophthalmologica, 90, s249 (2012). DG Dodwell and DA Krimmel. Invest Ophthalmol & Vis Sci, 53 (2012). A Capone et al., Retina, 34, 342–351 (2014). PMID: 23846381. P Dugel et al., Paper presented at the American Academy of Ophthalmology (AAO) annual meeting; November 14–17, 2015; Las Vegas, USA. S Yang et al., Drug Des Develop Ther, 10, 1857–1867 (2016). PMID: 27330279.
