- Although pharmacotherapy for DME has been successful, frequent injections are a drawback
- With current micropulse laser therapy (MPLT), thermal damage to cells is no longer an issue
- MPLT modulates the secretion of multiple cytokines by the retinal pigment epithelium
- A combination of treatment modalities for DME could be most effective
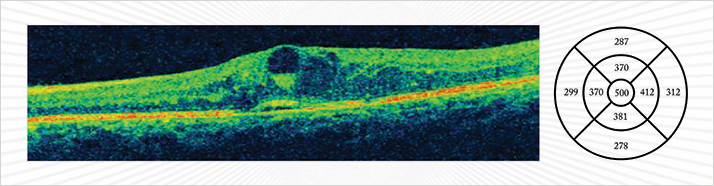
Last year, the International Diabetes Federation estimated that 55.2 million adults in Europe – which is 8.5 percent of the population – have diabetes. Over the course of their disease, a quarter of these patients will develop diabetic macular edema (DME; Figure 1). The pathogenesis of DME is multifactorial. It includes microvascular and neuroinflammatory alterations that result in increased vascular permeability or ischemic changes.
Last year, the International Diabetes Federation estimated that 55.2 million adults in Europe – which is 8.5 percent of the population – have diabetes. Over the course of their disease, a quarter of these patients will develop diabetic macular edema (DME; Figure 1). The pathogenesis of DME is multifactorial. It includes microvascular and neuroinflammatory alterations that result in increased vascular permeability or ischemic changes.
The history of DME treatment
Laser photocoagulation was essentially the first effective DME therapy. The cornerstone project for this was the Early Treatment of Diabetic Retinopathy Study (ETDRS), which began in the late 1970s. The protocols developed in the trial had, until very recently, represented the standard of care for DME. ETDRS demonstrated the efficacy of focal laser photocoagulation in reducing moderate visual loss in clinically significant DME (1). However, grid laser treatment (although considered effective against diffuse DME) induces the formation of progressively expanding scars that actually decrease vision, causing subretinal fibrosis and visual field loss (2).The treatment protocol for DME has been revised in light of the advances in pharmacotherapy. While laser photocoagulation was effective at halting disease progression, therapies directed against vascular endothelial growth factor (VEGF) appeared to restore visual acuity. The RISE and RIDE studies demonstrated the efficacy of the anti-VEGF agent, ranibizumab. It restored upwards of 15 letters of visual acuity in a significant percentage of subjects (3), providing a viable treatment option for cases of diffuse DME. Later, the VISTA-DME and VIVID-DME trial one-year results were published that showed that the cohorts that received aflibercept achieved dramatically better results than the laser photocoagulation groups, with gains of 10 ETDRS letters or more (4). The initial shine of anti-VEGF treatment is, however, starting to lose some of its luster. The biggest drawback is the frequency of injections required. While pro re nata (PRN) protocols are popular, patients that receive injections monthly tend to get the best results. Monthly injections are costly and many patients greatly dislike receiving them; both factors contribute to a reduction in patient compliance.
There may also be other issues. Growing evidence points to key roles played by VEGF in a healthy functioning eye (5): it may be harmful to indiscriminately prevent the function of VEGF. In addition, studies comparing anti-VEGF therapy to laser photocoagulation have not taken in to account new laser modalities, which reduce side effects and improve visual function results.
Micropulse laser therapy
When it was shown that argon macular laser photocoagulation reduced moderate vision loss by 50 percent in the ETDRS study, it was thought that burning de-bulked the diseased retina, increased intraocular oxygen tension and altered the production of vasoactive cytokines, including VEGF (6). That view has since been revised. Modified laser treatments, such as micropulse laser therapy (MPLT; Figure 2) can avoid the retinal damage that was associated with the older laser technologies. These newer devices deliver laser energy in a more controlled manner, and are able to produce a chain of short pulses, separated by longer pauses: this allows the tissue to cool, preventing thermal buildup and the retinal damage that is associated with the heat.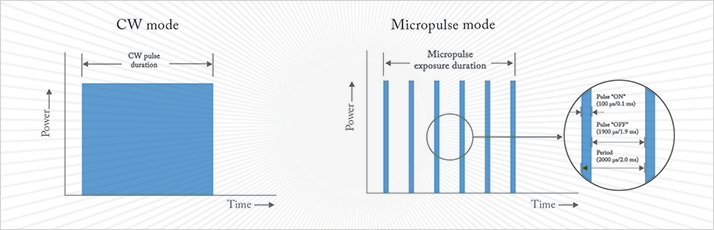
The intention was to decrease negative side effects such as the destruction of retinal photoreceptors, retinal scars, choroidal neovascularization and the development of macular scotomas, and the approach represented a welcome evolution in the application of laser therapy. The first study that evaluated MPLT in patients with DME was reported in 2005; the investigators wished to establish if the new technique could avoid laser-induced retinal injury (7). The results were comparable to conventional photocoagulation in terms of visual acuity and fluorescein angiographic leakage, but without the adverse effects associated with it: the approach represented a welcome evolution in the application of laser therapy. It also instigated a revolution in our understanding of the therapeutic mechanism that underlies laser treatment for DME.
My colleagues and I recently reviewed the literature on the topic (8) and offered an explanation for the mechanism of action of sub-threshold micropulse lasers in DME, namely alterations in the retinal pigment epithelium’s expression of cytokines. (The cytokines are a a family over 100 small cell-secreted proteins that affect the behavior of other cells.) An increase in the concentration of a variety of cytokines (including VEGF) has also been observed in the aqueous humor and the vitreous of patients with DME. Anti-VEGF treatment selectively inhibits just VEGF. Although VEGF plays an important role in both angiogenic and inflammatory pathways, selective anti-VEGF treatment is unlikely to influence other immunogenic cytokines involved in DME. On the other hand, a growing body of evidence indicates that low-intensity red and near-infrared laser promotes proliferation of multiple cell types, mainly through the activation of the mitochondrial respiratory chain and the initiation of cellular signaling.
MPLT using the IQ 810 or the IQ 577, (Figure 3, Iridex Corp, Mountain View, CA, USA) can induce favorable alterations in the expression of a large variety of potent extracellular mediators of DME, while avoiding any lethal thermal cellular damage. High-density MPLT maximizes the effective surface area and therefore the therapeutic effect. The small physiologic changes in cytokine expression resulting from MPLT may account for the slower onset and longer-lasting benefits observed following all types of laser treatment for DME. Although the slower reaction time could be considered a disadvantage of MPLT, it is compensated for by a longer-lasting effect and an excellent safety profile in comparison with other types of laser treatment. No retinal damage has been observed following MPLT with either yellow or infrared lasers, as demonstrated by color fundus photography, fundus autofluorescence (FAF) imaging, fundus fluorescein angiography (FFA) and spectral domain optical coherence tomography (SD-OCT) (10).

A combined treatment approach
In my practice, we evaluate whether or not the DME involves the center of the fovea: if it does not, we perform MPLT; if it does, we evaluate the central retinal thickness (CRT). In patients with CRT up to 400 µm, we perform MPLT. If CRT is greater than 400 µm, we first perform anti-VEGF injections and follow-up with MPLT after the edema has reduced below 400 µm. We do not see any visible scarring in our patients even after multiple retreatments, reassuring us of the safety of MPLT when performed at the lowest duty cycle. As there is an important inflammatory element to DME, we are also investigating a role for long-term steroid therapy. Slow-release corticosteroids have shown promising results and their use may be a valid option in specific DME phenotypes.Our understanding of both the disease processes that underlie DME and the impact of laser stimulation has dramatically improved, and we are now better able to define and evaluate treatment options. DME is a chronic disease that requires long-lasting treatment options that have minimal side-effects. To achieve this, there is still much to be investigated, such as a randomized trial of anti-VEGF combined with MPLT and a better understanding of which are the optimal cases for treatment with steroids. DME is a multifactorial disease that undoubtedly will benefit from a multi-faceted treatment approach, and time will teach us how to match protocols and disease profiles. Stela Vujosevic is the Assistant Clinical Professor of Ophthalmology at the University of Padova, Italy.
References
- Early Treatment Diabetic Retinopathy Study research group, “Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1”, Arch. Ophthalmol., 103, 1796–1806 (1985). G.G. Striph et al., “Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field,” Ophthalmology, 95, 1673–1679 (1988). Q.D. Nguyen et al., “Ranibizumab for DME: Results from 2 phase III randomized trials: RISE and RIDE,” Ophthalmology, 119, 789–801 (2012). doi:10.1016/j.ophtha.2011.12.039. U. Schmidt-Erfurth, “Efficacy and safety of intravitreal aflibercept in DME: results of two phase III studies (VIVIDDME and VISTA-DME).” Paper presented at: the 13th EURETINA Congress; September 27, 2013; Hamburg, Germany. M. Saint-Geniez, P. D’Amore, “VEGF Has Physiological as Well as Pathological Functions,” The Ophthalmologist, 1, 16–23 (2014). M.A. Mainster, “Decreasing retinal photocoagulation damage: principles and techniques”, Semin. Ophthalmol., 14, 200–209 (1999). J.K. Luttrull, D.C. Musch, M.A. Mainster, “Subthreshold Diode MicroPulse Photocoagulation for the Treatment of Clinically Significant Diabetic Macular Oedema”, Br. J. Ophthalmol, 89, 74–80 (2005). S. Vujosevic et al., “Subthreshold Laser Therapy For Diabetic Macular Edema: Metabolic And Safety Issues,” Curr. Med. Chem., 20, 3267–3271 (2013). J.K. Luttrull, “Subthreshold diode MicroPulse photocoagulation for the treatment of clinically significant diabetic macular oedema,” Br. J. Ophthalmol. 89, 74–80 (2005). doi: 10.1136/bjo.2004.051540. S. Vujosevic et al., “Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold MicroPulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation”, Retina, 30, 908–916 (2010). doi: 10.1097/IAE.0b013e3181c96986.
