
Every innovation should be preceded by a need. As with most surgeons, I always contemplate the wants and needs of my patients: how can their safety be enhanced? How can we make them more comfortable? Can their surgery be performed more efficiently or effectively? Such questions have motivated me to embark upon journeys of creativity, invention, and – to overcome inevitable hurdles – learning opportunities. Ultimately (or hopefully!), no matter how rocky the road, the destination is an innovation that fulfils the original need.
I’ve had the good fortune to be involved in the development of two innovations: the Waqar suture removal forceps and the intravitreal (IVT) injection guide (Malosa single use instruments, BVI). And I’ve also had the good fortune to be in a supportive workplace and to have a framework around me that has enabled me to divert my time and energy. But this isn’t the case for everyone; constraints or a lack of encouragement can stop many in their tracks. Here, I hope to encourage would-be innovators to bring their ideas to life by providing my own personal guide to getting an innovation off the ground.
The journey from idea to market
Step 1: The birth of an idea
First, put your idea down on paper; sketch out the skeleton, including the main concepts and features. Sign and date it; this will act as proof of originality if issues of patents or intellectual property (IP) arise further down the line.
Step 2: Understand who holds the IP rights
As I am an employee of the National Health Service (NHS) in the UK, my employer holds the IP rights to any idea relating to my routine work. So, in establishing IP ownership, your hospital must be your first point of contact. This process may vary in different countries, so it is useful to know what the unique arrangements are in your place of work. Developing good working relationships helps greatly at this stage.
Step 3: Keep it to yourself
Don’t disclose your idea or you may affect subsequent patent applications.
Step 4: Call in the help!
If your workplace permits, get in contact with the local innovation team – which may include someone with an engineering or IP background – as well as the research and development (R&D) team, to gain their insight. For me, this is the NHS innovation panel and pathway team, and a regional pathway, which is run independently as a collaboration between local R&D departments. This framework was able to provide much needed expertise in all areas ranging from computer-aided designs (CAD), 3D printing and IP, to negotiating contracts with commercial companies.
Step 5: Be patient
Patience is key during the planning and modeling phase; our in-house experts made several different models of the IVT injection guide before achieving the final version.
Step 6: Find a commercial partner
It is essential to find a supportive commercial partner to manufacture, market, and distribute your product – for me, that was BVI. The right commercial team will fully understand the nuances of every stage of the development process, as well the legal aspects, such as non-disclosure agreements and IP, and they will propose how your product can be cost-effectively manufactured to scale.
Bringing ideas to life
The Waqar suture removal forceps (see Figure 1) were created to ease the removal of corneal sutures and to improve patient safety. Instead of the traditional two-step technique – using a long 23- or 25-gauge needle with separate forceps, which can pose safety issues in the hands of inexperienced surgeons or nurse practitioners – the new instrument eliminates the sharp, long needle, making suture removal a one-step process.
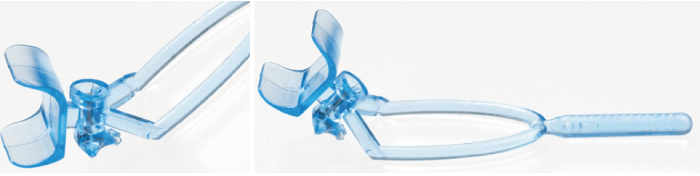
Patient safety and comfort were also at the forefront of my thinking in designing the IVT injection guide (in collaboration with Plymouth and Torbay NHS innovation panels; see Figure 2). The guide was created to facilitate the fast, safe, and precise delivery of an IVT injection.
With the prevalence of retinal disease continuously increasing, the growing volume of IVT injections is placing substantial pressure on medical retina departments. In short, the quicker IVT injections and other services can be delivered without compromising safety or quality, the better. The traditional way to deliver an IVT injection involves applying a lid speculum (which can be quite uncomfortable), and a surgical drape across the patient’s face (which can feel claustrophobic). Finally, a caliper is used to measure the site of the injection. The idea was to create an instrument that would combine these three steps, while controlling position, depth, and the angle of injection to improve efficiency and increase surgical throughput.
The guide consists of a curved, triangular base plate, which follows the natural contour of the eye, and three studs at each corner, which ensure stabilization. The cylindrical chamber adjacent to the lash guard allows the injection to be delivered at the precise location and depth of the eye, avoiding the risk of retinal damage. All these features were given a great deal of consideration along the developmental pathway, but getting them finalized did not come without challenges...
Not plain sailing
For any clinician, taking an idea further can be daunting and difficult. The key is to know what resources to use. As I mentioned earlier, I can’t emphasize enough the importance of having a supportive and informative team around to guide you; it will make any difficulties that arise easier to overcome.
One big challenge for me was simply finding time. As a clinician, time is already in short supply; work, family life, and other commitments leave little room for innovation. You really need to rely on your drive and passion.
You must also prepare to face skepticism. During the development of both the Waqar suture removal forceps and the IVT injection guide, many clinicians were skeptical about their effectiveness. To date, we have published evidence that demonstrates the safety and efficacy of the IVT injection guide (1). In this paper, we presented results from two sites (the first comparing outcomes in 50 patients, 25 of whom received an IVT injection with the guide and 25 without; the second focused on retrospective notes evaluation of 60 patients injected with the device). No adverse events were noted, and the device appears to be well tolerated by patients. Use of the guide did not result in a statistically significant increase in pain (p value >0.05), but the mean score (on a unidimensional numerical rating scale) was noted to be slightly better. All patients gave very positive, informal feedback.
We also have anecdotal evidence of efficiency benefits from nurse practitioners in Plymouth, UK, where they report a higher throughput of patients when using the guide to deliver an injection compared with the traditional method of delivery. We are now doing a time and motion study to objectively quantify this and expect more evidence in the next year or so.
The surgeon knows best
To conclude, I’d like to say how important I think it is for innovations to be driven by surgeons. Clinical experience is key; it is only the surgeon who knows what works, what doesn’t, and what is most likely to improve the lives of their patients.
The short version for budding innovators: be motivated from the start and maintain the momentum. Set aside time and be realistic about the amount of time required. Don’t cut corners; go through each step of the development process to improve your chances of developing a valid end product. And don’t forget to have fun!
References
- S Waqar et al., “A novel device for rapid, safe and precise delivery of intravitreal injections”, BMJ, 5, 8 (2019).
