“What does a transcription factor expressed in the cornea have to do with glaucoma?” asks Eldon Geisert Jr, Professor of Ophthalmology at Emory Eye Center, Atlanta, Georgia, USA. Well, Geisert and his team have identified that the transcription factor in question – POU6F2 – could be a risk factor for primary open angle glaucoma (POAG) (1). Thinner corneas are a well-known risk factor for POAG, but no one has really understood how or why, because of the multiple confounding genetic and environmental factors – and the need for extremely large sample sizes for genetic analysis. Inspired to understand more about the mechanisms of neuronal death in glaucoma, Geisert and his team set out to investigate the potential molecular link between central corneal thickness (CCT) and this process with a more feasible approach.
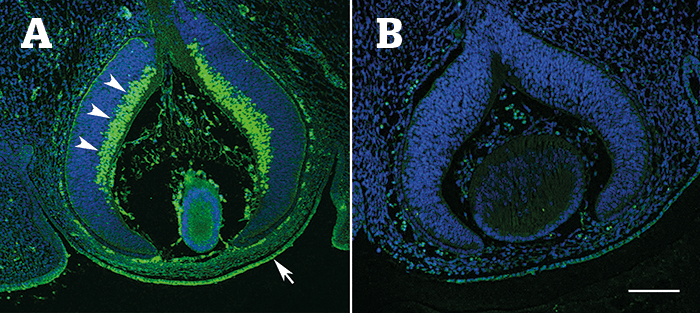
Using BXD RI mice (strains of fully mapped and sequenced inbred mice that allow genetic loci to be linked to phenotype), the team measured the CCT of 818 mice from 61 members of the strain set, and used the information to identify novel quantitative trait loci (QTLs) that modulate CCT with bioinformatics data analysis tools hosted on GeneNetwork.org. Comparing the candidate genes from this analysis with human corneal and glaucoma genome-wide association (GWAS) datasets, the team identified that the top 50 hits in the POAG dataset resided in the locus for POU6F2. Studying POU6F2 expression in embryonic mice, the team identified that the transcription factor was strongly expressed in neuroblasts – precursors to retinal ganglion cells (RGCs) – as well as in developing corneal endothelium and corneal stem cells (Figure 1). In adult mice, POU6F2 was strongly expressed in a subset of RGCs. The team also identified that CCT was significantly thinner in Pouf62--null mice compared with wild-type littermates (p<0.01), and that POU6F2-expressing RGCs were susceptible to death in a mouse model of glaucoma (1).
“As almost everyone believes that the link between CCT and glaucoma is due to the stiffness of the cornea and the sclera, the associated genes should be associated with extracellular matrix or collagen – not a transcription factor,” says Geisert. “In our case, the results did not fit the current paradigm.” Explaining that POUF62 has completely different roles in the different tissues of the eye, Geisert says: “In the cornea, POU6F2 is involved in development of the tissue and marks the stem cells that maintain corneal integrity; in the retina, it is part of a molecular signature that modulates the susceptibility of the RGCs to injury.” The team believes that their identification of POU6F2 as a potential risk factor for POAG could not only provide a marker for the early detection of POAG, but also further the understanding of why some retinal cell types are particularly sensitive to injury. Geisert also hypothesizes that POU6F2 could also be a risk factor for normal tension glaucoma. “We also study ocular blast injuries for the Department of Defense, and in this model of ocular injury, the POU6F2-expressing cells appear to be the first to die.”
References
- R King et al., “Genomic locus modulating corneal thickness in the mouse identifies POU6F2 as a potential risk of developing glaucoma”, PLOS Genet, 14: e1007145 (2018) PMID: 29370175.
