- Goldmann applanation tonometry (GAT) is known to return clinically significant IOP reading errors in about half of patients, resulting in suboptimal clinical decisions – and even glaucoma misdiagnosis
- Accurate GAT tonometry requires that IOP readings are corrected to allow for corneal biomechanical variations associated with measurement error, but to date this has been impractical with the exception of incomplete CCT correction
- We used advanced mathematical modeling to design an optimized correcting applanation tonometry surface (CATS) for the existing Goldmann tonometer interface to minimize IOP errors
- Clinical studies have demonstrated that the CATS prism reduces IOP measurement errors to below ± 2 mmHg in 98 percent of patients, compared to only 50 percent with the existing GAT.
The critical task of measuring IOP lies with a 60-year-old method so error-prone that relying on its readings may be sight-threatening for many patients. How did we come to rely so heavily on Goldmann applanation tonometry (GAT)? Fundamentally, GAT’s problem stems from its assumption that the cornea is an infinitely thin membrane requiring no force to applanate – other than that produced by the IOP, which is simply not true. In fact, biomechanical variations in the cornea modulate the applanation forces acting on the tonometer prism, and these force variations are then incorrectly interpreted by GAT as IOP variations. In fact, fully 50 percent of patients deviate from ‘nominal’ cornea sufficiently to induce clinically significant inaccuracies in GAT IOP readings. So although GAT may provide accurate measurements for patients with ‘nominal’ corneas, many others are failed by the technique.
Corneas may diverge from the ‘nominal’ in four main aspects: thickness, rigidity, curvature and tear film. In particular, IOPs are often over-estimated in eyes with thick corneas and under-estimated in eyes with thin corneas (1); this in itself can result in glaucoma misdiagnosis. Measurement errors can also occur with steeply curved corneas; these will be significantly over-estimated during GAT. Similarly, flat corneas (commonly found after LASIK surgery) are associated with IOP under-estimations. Even the tear film can have an effect – inter-patient variations in the adhesion forces associated with tear film surface tension will confound true IOP measurement. These issues have been recognized for many years. Proposed remedies have included measuring and correcting for central corneal thickness (CCT); however, this – albeit the current standard of practice – addresses only one of several error sources, and therefore is at best an incomplete correction. Conversely, multiparameter algorithms, intended to correct the effects of multiple error sources, have proved too cumbersome for clinical use. In summary, the situation is highly unsatisfactory – but how can it be addressed?
Model answers
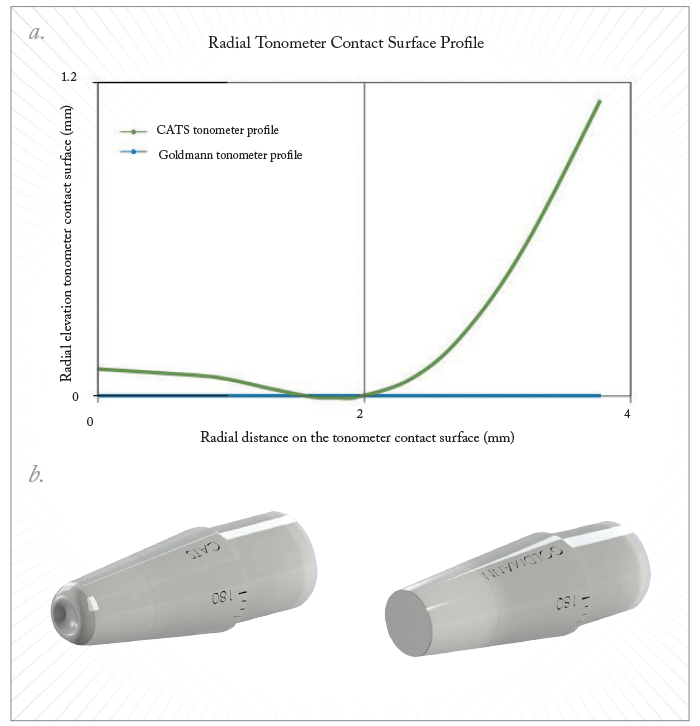
Our solution was to develop CATS – a tonometer prism that automatically corrects for the major sources of corneal variation. In brief, development required i) detailed mathematical modeling to predict the optimal topology for the prism surface; ii) construction and validation of prism prototypes; and iii) testing CATS in clinical studies. The first step was to develop a mathematical model of the cornea. Non-linear, hyperelastic finite element modeling (FEM) simulations allowed us to analyze effects of corneal properties on IOP measurement, which in turn made it possible to design a prism surface that would – in theory – improve measurement accuracy (2). We used our model to flatten the (simulated) IOP isobaric curves with respect to error-generating corneal parameters and thereby derive an optimized topology for the prism surface (Figure 1).According to our model, the optimal prism surface should not be flat (as in GAT) but should be comprised of a polynomial-approximated central concavity surrounded by a convex annular rim. Such topology was predicted to allow the prism to minimize the intra-corneal stress as well as tear-film adhesion, and thereby reduce each measurement error by ~50 percent. The next step was to manufacture and validate prototypes of the optimized prism. Accordingly, CATS prototypes were inserted into standard Goldmann tonometer armatures and used to measure IOP in cadaver eyes (2). The method, in brief, used a specially designed chamber (Figure 2) to stabilize enucleated globes while IOP measurements were taken by GAT, CATS and manometry. The object was to achieve a prism design that gives the same pressure reading in a ‘nominal’ cornea as GAT. This bench testing period was also intended to demonstrate accuracy and repeatability of IOP measurements: specifically, to test IOP measurement bias and variability by comparing CATS and GAT prism measurements relative to true IOP values as given by manometric pressure transducers. We were delighted to find that, as predicted by our model, the optimized CATS prism was substantially equivalent to GAT in eyes with nominal corneal parameters (2).

The final step was to investigate CATS performance in patients with varying corneal parameters. Initial clinical studies aimed to identify any biases. Each time we found a bias, we optimized the prism design and went back to patients with the new models; we went through three such iterations. Next, we assessed the ability of the optimized CATS system to correct for identified sources of IOP measurement error. Comparing tonometer-measured IOPs with readings from intracameral transducers is difficult in patients, so for our first study (3), we opted to compare GAT and CATS readings, and to correlate corneal variability with any differences identified in the GAT versus CATS comparison. Specifically, we looked at the following corneal parameters: CCT, corneal resistance factor measured by the ocular response analyzer, and corneal curvature. (We did not assess corneal tear film, as this parameter is better suited to measurement under the static conditions of cadaver eyes). After analyzing data from 109 eyes, the findings were clear (3): IOP measurement differences between CATS and GAT were evident, and correlated with known error parameters (corneal thickness, stiffness and curvature). The effect was very obvious: for CCT, for example, whereas IOP measurement inaccuracies of >±2 mmHg were found in 46 percent of all patients when using GAT, IOP measurement errors of >±2 mmHg were found in only 3 percent of patients when using the CATS prism (3). Overall, as predicted by our model, CATS is associated with ~50 percent reductions in errors related to CCT, corneal rigidity and curvature variations.
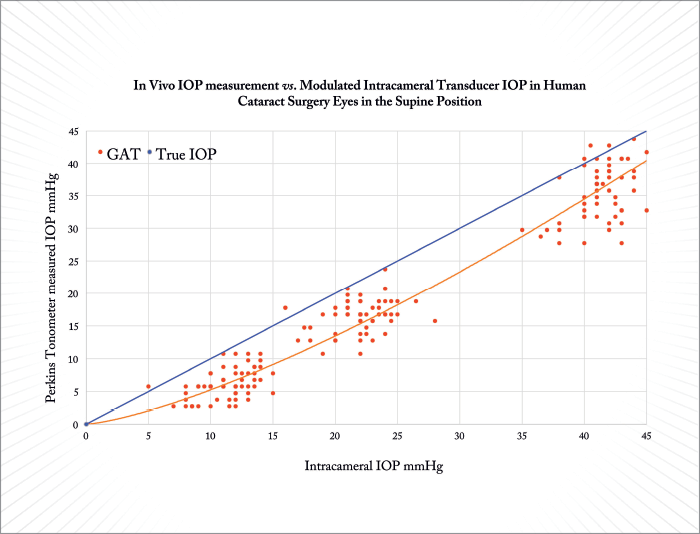
One limitation of the study outlined above was its lack of a comparison between tonometric IOP readings and true intracameral pressure readings. We have now remedied this with a follow-up study (4) that shows similar findings to the initial study: in short, we assessed the correlation between GAT errors and corneal variations in cataract surgery patients, and performed additional cadaver eye studies. Prior to surgery, we measured CCT and CRF with an ocular response analyzer. During surgery, we modulated the pressure on the inside of the eyes sequentially to 10, 20 and 40 mmHg, using a bottle height-modulated intracameral transducer, and simultaneously measured the IOP from outside to assess the accuracy of the tonometers. Finally, we corroborated our findings in 21 fresh cadaver eyes, with IOPs modulated between 5 and 60 mmHg, again comparing tonometer-measured pressures with those reported by an intracameral pressure transducer inside the eye. Encouragingly, we found that CATS gave very repeatable measurements, both within and between practitioners. We also found that GAT significantly underestimated IOP (by 5.2±1.6 mmHg – Figure 3), and that this underestimation was greater for patients in the supine position (7.9±2.3 mmHg). These errors were highly correlated with patient CCT. Thus, the results from patients both corroborated in vitro data and validated our mathematical modeling.
CATS out of the bag
It is now clear that a CATS prism permits more accurate IOP measurement – and it does so without requiring any changes to the standard GAT technique. All practitioners to date have easily accommodated to the CATS prism: it really is just a case of taking the GAT prism off and putting the CATS prism on, and needs no additional examination time or specialized data interpretation. Another significant user benefit of CATS relates to centration. GAT will measure applanated mires at any point on the flat prism surface – but precise IOP measurement requires the cornea to be accurately centered on the prism face, which is subjective and so constitutes another source of error. CATS is an autocentrating system; the mires line up exactly only when the cornea is centered on the applanation surface. Hence, it is more difficult to take a inaccurate CATS measurement! What will these advantages mean once CATS is approved and released on to the market? We will soon find out – we have completed our clinical trial for FDA clearance, and hope to reach the market in early 2018. When we do, there will be real benefits for patients. For example, I recently tested a glaucoma suspect with an increased cup-to-disc ratio and a CCT of 490 microns; the IOP as measured by GAT was only 16, but with CATS the pressure reading was 22. Detecting such significant IOP elevations will have profound implications for many patients. Groups that may particularly benefit include those with low pressures, say around 10 mmHg, after stent or trabeculectomy surgery – such low pressures are often read incorrectly. In fact, at 5 mmHg, the GAT system may give you a zero reading! But CATS can still measure pressure accurately at that level, perhaps because the cornea is not buckling, as it does with GAT, but is conforming to the prism surface. So the greater accuracy of CATS will change clinical decisions for the better – the IOP measurement is ‘real’, and requires neither interpretation nor correction.CATS eyes up the future
In the immediate future, CATS will be easily and broadly adopted: it is compatible with the in-office GAT systems used by virtually all ophthalmologists, and its operation is identical to the GAT device. Also, there are no hidden expenses or inconveniences associated with CATS: it doesn’t disrupt patient flow, and doesn’t need maintenance or user training. Indeed, CATS could be cost-saving in that it will eliminate the need to purchase, maintain and use expensive pachymeters (as it already corrects for CCT). In the longer term, we expect the device to help in the assessment of: i) patients on eye drops who perhaps should not be on drops; ii) patients with progressive visual loss; and iii) specific populations including keratoconus patients, pediatric populations, post-LASIK patients and even veterinary indications. First versions of the device will likely be reusable units; later iterations will most likely have the added convenience of being disposable. In summary, compared with GAT our CATS prism detects higher IOPs in eyes with thin corneas, and lower IOPs in eyes with thick corneas; higher IOPs in less rigid corneas, and lower IOPs in more rigid corneas; higher IOPs in flatter corneas, and lower IOPs in steeper corneas. It does this with high repeatability and without detectable bias, and should result in better clinical decisions for many patients. Clinical data (3) validate our mathematical modeling approach (2). Finally, CATS couldn’t be simpler or easier to implement – yet its impact will be dramatic; it will allow accurate measurements of IOP in the 50 percent of the population who do not have a ‘nominal’ cornea and are, therefore, not adequately served by GAT. And that’s a lot of people! Sean McCafferty is Founder, President and CEO of Intuor Technologies, LLC. After an undergraduate degree in mechanical engineering, he worked as a product development engineer before attending Ohio State University’s Medical School and completing a residency in ophthalmology at the University of Arizona. Subsequently, he completed an MSc in optical engineering expressly to allow his ideas to become reality in terms of products for the ophthalmic industry.References
- M Kass et al., “The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma”, Arch Ophthalmol, 120, 701–713 (2004). PMID: 15197055. S McCafferty et al., “Goldmann tonometer prism with an optimized error correcting applanation surface”, Transl Vis Sci Technol, 5, 1–13 (2016). PMID: 27642540. S McCafferty et al., “Goldmann tonometer error correcting prism: clinical evaluation”, Clin Ophthalmol, 11, 835-840 (2017). PMID: 28496302. S McCafferty et al., “Goldmann applanation tonometry error relative to true intracameral intraocular pressure in vitro and in vivo”, BMC Ophthalmol, 25, 215 (2017). PMID: 29178849.
