Experts predict that glaucoma – the second leading cause of global blindness – will affect 79.6 million people by 2020 (1). Lowering IOP is currently the only proven method for preventing or slowing down the condition. And that’s why it is crucially important that ophthalmologists are able to accurately detect fluctuations in eye pressure to ensure treatments are effective – and that patients are complying with recommended regimens. However, the time, effort and cost of IOP monitoring mean that it’s not possible for ophthalmologists to measure IOP with optimum frequency in the clinical environment, during office hours. Does this matter? Yes – given the high probability that important IOP peaks could be missed, leading to a worsening of the condition.
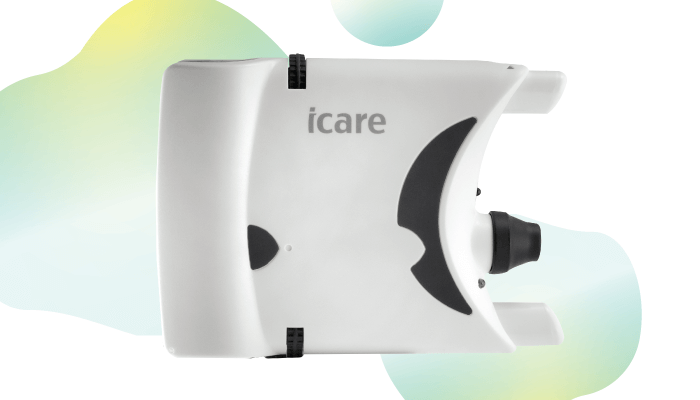
The Icare HOME tonometer offers patients and ophthalmologists an easy-to-use method for 24-hour IOP monitoring outside of the office environment, providing doctors with real-world diurnals in as little as a week. Developed by Icare Finland, a global leader in handheld tonometry, the device uses a rebound technique for measuring eye pressure that is barely noticeable; a very light, sterile probe makes momentary contact with the cornea, and then rebounds. The technology makes the Icare HOME the first tonometer that does not require any anesthetic, drops or air puffs for use outside the doctor’s office. The HOME measures the deceleration of the probe and contact time, and uses a patented algorithm to calculate the IOP measurement. This puts the technology of the Icare clinical tonometer in the hands of a patient. Afterwards, the user can send the IOP measurements to a healthcare professional via the Icare CLINIC in a secure HIPAA compliant cloud for review and analysis.
The device includes automatic OD/OS recognition technology – an intelligent positioning assistant that guides correct alignment of the tonometer – and an automatic measurement sequence feature that can switch between “series” and “single” modes. Approved by the FDA over two years ago, the Icare HOME now qualifies for reimbursement via remote physiological monitoring. CMS classify these as permanent category 1 codes.
Regular – at-home – monitoring provides more reliable IOP information to the ophthalmologist, enabling them to better personalize treatment to each patient. The ophthalmologists benefit from improved clinical efficiency, and the treatment process is more convenient for patients. Moreover, by putting tonometry into the hands of patients, the Icare HOME could help improve patient engagement and therefore increase treatment compliance.
For more information, see the FAQ: www.corcoranccg.com/products/faqs/rpm-icare-home-tonometer-icare
References
- HA Quigley and AT Broman, “The number of people with glaucoma worldwide in 2010 and 2020”, Br J Ophthalmol, 90, 3 (2006). PMID: 16488940.
