- Advanced keratoconus can be managed and treated with many methods – but all have drawbacks: the challenge is to minimize them
- Keratoplasty is an option of last resort – but PK and DALK sacrifice much of the host cornea
- Bowman’s layer transplantation (BLT) and placement into a mid-stromal pocket is a potentially tissue-sparing approach. It might restore some corneal architecture – but it doesn’t address the primary problem of apical stromal thinning
- We describe a mid-stromal lamellar keratoplasty technique (MSLK) that both increases central corneal bulk and thickness, and flattens the cornea more than BLT, and describe the first clinical application of MSLK
There are several ways to treat keratoconus today, but none are perfect – each approach comes with drawbacks or limitations. Take corneal collagen cross-linking, which has revolutionized the field because of its ability to strengthen the cornea and slow progression (1) – and even flatten it slightly (2). But it’s never going to restore the corneal architecture, so your patients’ often highly debilitating visual symptoms remain.
You do have a number of strategies available to improve your patients’ visual acuity (VA), starting with spectacle correction and moving onto rigid gas permeable contact lenses (RGPCL), intra-corneal ring (ICR) segments and phakic toric intraocular lenses (IOLs) (2). But again, there are drawbacks: people can become RGPCL-intolerant, ICR segments flatten the mid-periphery and have a variable effect (especially if the ectasia is primarily central), and phakic IOLs only correct regular astigmatism. In more advanced disease (or in cases of RGPCL intolerance), you then have to consider penetrating keratoplasty (PK) or deep anterior lamellar keratoplasty (DALK) – but this approach sacrifices the majority of the host cornea. Other older keratoplasty techniques have fallen out of favor – but keratoplasty for the treatment of corneal ectatic disorders, such as keratoconus, is an area of intense research. For example, Gerrit Melles’ team has recently described Bowman’s layer transplantation (3), which involves the isolation and detachment of Bowman’s layer from the anterior stroma of a donor cornea and transplantation into a manually created mid-stromal pocket. Why? Histopathological studies have indicated that Bowman’s layer fragmentation contributes to the progression and visual debilitation of keratoconus (4), so its replacement is a logical therapeutic approach. However, the fragmentation of Bowman’s layer is a late and secondary phenomenon in keratoconus, and there’s little or no established correlation between its fragmentation and reductions in VA (5). Replacement tissue will restore some of the original shape of the cornea, but it does not address the primary problem of apical stromal thinning – one of the biggest contributors to the corneal protrusion and irregular astigmatism present in keratoconus. Histopathological studies have shown that this stromal thinning is caused by a significant increase in the diameter of the collagen fibrils in the stroma and their interfibrillary distance (6), alongside a reduction in their number (7). In theory, a procedure involving an intrastromal lamellar graft would, therefore, be expected to not only increase the central corneal bulk and thickness but also flatten the corneal architecture to a greater extent than Bowman’s layer transplantation – thereby reducing the need for more conventional grafts such as DALK or PK. We report the first case of a novel surgical approach in the form of a mid-stromal lamellar graft assisted by collagen cross-linking for the management of advanced keratoconus. Whilst small incision lenticule extraction with cross-linking has been used for the treatment of keratoconus (8) this is the first report, to our knowledge, of an intrastromal lenticule being implanted to restore the stromal architecture in a keratoconic cornea.
Table 1. Key corneal parameter assessments, pre- and post-operatively (up to four weeks’ follow-up). UCVA; uncorrected visual acuity. BCVA; best corrected visual acuity. CCT; central corneal thickness. IOP; intra-ocular pressure. GAT; Goldmann applanation Tonometry
Methods
Our patient was a 28 year old with advanced keratoconus and RGPCL intolerance. Following informed consent, a number of preoperative measurements were obtained including pachymetry, topography, anterior segment OCT (AS-OCT), and intraocular pressure measurements with Goldmann applanation tonometry and iCare tonometry. The lamellar graft/lenticule was prepared with a Gebauer SLc Expert microkeratome system. This keratome, plus the use of a pre-shaped base, allowed precise cuts of defined thickness and a pre-defined shape to be made. This permitted the definition of two separate parameters: for this patient, a thickness of 100 µm with a 7 mm diameter, and a planar rather than concave or convex shape was chosen. An anterior chamber paracentesis was created at 9 o’clock and air was injected following aqueous aspiration. A 7 mm superior limbal incision was fashioned to a depth of 250 µm and a mid-stromal pocket was then created manually using the dissection technique previously described for DALK (9), encompassing a diameter of 8 mm. The lamellar graft was guided into the stromal pocket with an anterior chamber IOL surgical glide and positioned with a Rycroft anterior chamber cannula. Cross-linking was performed by immersing the intrastromal pocket (and graft) in riboflavin for 10 minutes followed by ultraviolet light exposure (9 mW) over a 9 minute period. Post-operative anterior segment photographs, AS-OCT images, and topography are highlighted in Figures 1–3.Results
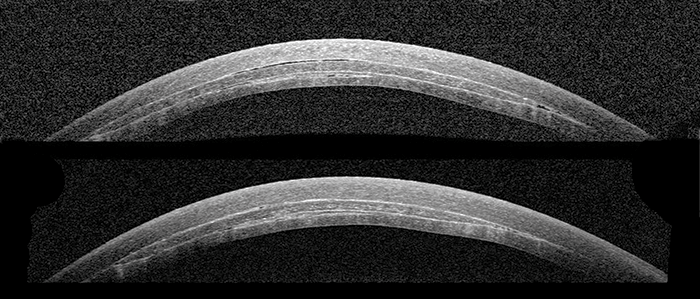
Post-operatively, there was a significant reduction in topographic cylinder over four weeks and an increase in central corneal thickness of about 100 µm. The AS-OCT images show a well-positioned, mid-stromal lamellar graft (Figure 1). There is evidence of interface fluid, which would be expected to resolve with time and thereby improve contact and regularity between the graft and host surfaces, and this should aid further visual recovery. Table 1 details the patient’s pre- and post-operative results up to four weeks of follow-up.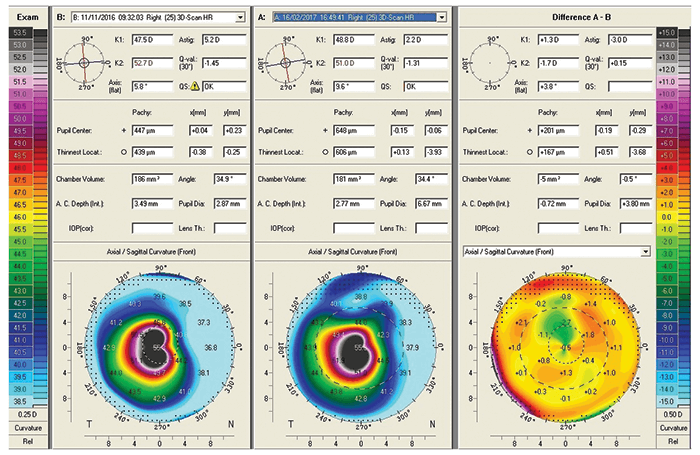
Discussion
Our technique theoretically confers a number of advantages over Bowman’s layer transplantation. First, the 100 µm planar lamellar button resting intrastromally would be expected to provide more strength, bulk and flattening of the corneal architecture than Bowman’s layer alone (which is approximately 17 µm thick (10)). In Bowman’s layer transplantation, the preparation of the graft involves manual dissection of Bowman’s layer with a 30-gauge needle and a custom-made stripping device as well as McPherson’s forceps. Given the delicate nature of Bowman’s layer, it is not surprising that tearing of the graft is a significant problem during preparation – this affects almost 30 percent of all grafts harvested (11). Due to its elasticity, Bowman’s layer also tends to roll up and needs to be unfolded manually within the stromal pocket, putting the graft at further risk of damage (11). The lenticule used in our technique is much thicker (100 µm) and is prepared using an automated microkeratome. It also includes Bowman’s layer within the lenticule, so it may have the benefits of Bowman’s layer transplantation, plus added bulk. In theory, this should make it less likely to be damaged during harvest.Our mid-stromal lamellar keratoplasty (MSLK) procedure has the advantage of being less technically challenging than Bowman’s layer transplantation and therefore is likely to have a more favorable learning curve – for example, it uses a microkeratome system to dissect the donor tissue, and only the host corneal pocket is created manually. There’s another potential advantage to using a microkeratome when performing the graft dissection – in DSEK, VA recovery is reported to be faster than when manual graft dissection is performed (12), likely secondary to a more irregular interface between the host and graft that’s created in manual dissection (13). The procedure may be improved further by femtosecond laser creation of the pocket. The relative absence of sutures (when compared with other techniques such as DALK and PK) means that MSLK is relatively less time-consuming: this first case took 45 minutes to complete.
There are a number of potential limitations of this technique, like intraoperative perforation of Descemet’s membrane, as has been reported with Bowman’s layer transplantation (3). It is likely that patients with a very thin cornea could be ineligible for MSLK as the risk of perforation may be high. However, the procedure could still be attempted and converted to a different form of keratoplasty if a perforation occurred, as in DALK. In addition, the procedure could be completed even in the presence of a perforation. A DALK or PK is likely to be advantageous in cases of significant corneal scarring involving the visual axis.
Conclusion
There are many methods by which keratoconus can be treated – but all have drawbacks associated with their use. Recent years have seen some innovative keratoplasty approaches that aim to minimize these drawbacks, and MSLK, it is hoped, offers an exciting way forward for the management of keratoconus, with fewer drawbacks and compromises than the Bowman’s layer transplantation approach – and might offer a viable alternative to DALK or PK.Mohammad Khan is a Corneal Fellow, Priscilla Mathewson is a Specialist Registrar, and Sunil Shah is a Consultant Ophthalmologist at the Birmingham Midland Eye Centre, Birmingham, UK. Jonathan Martin is a fourth-year medical student at the University of Bristol. The authors report no financial disclosures related to any product or technology mentioned in this article.
References
- G Wollensak et al., “Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus”, Am J Ophthalmol, 135, 620–627 (2003). PMID: 12719068. V Jhanji, N Sharma, RB Vajpayee, “Management of keratoconus: current scenario”, Br J Ophthalmol, 95, 1044–1050 (2011). PMID: 20693553. K Van Dijk et al., “Bowman layer transplantation to reduce and stabilize progressive, advanced keratoconus”, Ophthalmology, 122, 909–917 (2015). PMID: 25596620. K Van Dijk et al., “Midstromal isolated Bowman layer graft for reduction of advanced keratoconus”, JAMA Ophthalmol, 132, 495–501 (2014). PMID: 24557359. M Kaas-Hansen, “The histopathological changes of keratoconus”, Acta Ophthalmol (Copenh), 71, 411–414 (1993). PMID: 8362646. A Patey, M Savoldelli, Y Pouliquen, “Keratoconus and normal cornea: a comparative study of the collagenous fibers of the corneal stroma by image analysis”, Cornea, 3, 119–124 (1984). PMID: 6536429. Y Pouliquen, “Keratoconus”, Eye (Lond), 1, 1–14 (1987). PMID: 2951280. EO Graue-Hernandez et al., “Combined small-incision lenticule extraction and intrastromal corneal collagen crosslinking to treat mild keratoconus: Long-term follow-up”, J Cataract Refract Surg, 41, 2524–2532 (2015). PMID: 26703503. GR Melles et al., “A new surgical technique for deep stromal, anterior lamellar keratoplasty”, Br J Ophthalmol, 83, 327–333 (1999). PMID: 10365042. C López de la Fuente et al., “Evaluation of total corneal thickness and corneal layers with spectral-domain optical coherence tomography”, J Refract Surg, 32, 27–32 (2016). PMID: 26812711. EA Groeneveld-van Beek et al., “Donor tissue preparation for Bowman layer transplantation”, Cornea [Epub ahead of print], (2016). PMID: 27362885. MO Price, FW Price, “Descemet’s stripping with endothelial keratoplasty. Comparative outcomes with microkeratome-dissected and manually dissected donor tissue”, Ophthalmology, 113, 1936–1942 (2006). PMID: 16935344. DR Anijeet, D Rachdan, S Shah, “Visual improvement after corneal endothelial transplantation: are we seeing better?”, Br J Ophthalmol, 96, 309–310 (2012). PMID: 22296833.
