Retinoblastoma is the most common primary intraocular cancer in children, accounting for 12 percent of all infantile cancers each year. There’s a high cure rate – of between 95 and 98 percent – and therapies include laser thermotherapy, cryotherapy, radioactive plaques, external beam radiotherapy, chemotherapy, and ultimately, enucleation. Sadly, radiotherapy and chemotherapeutic approaches can lead to treatment-related secondary cancers, so there’s certainly a need for a new approach that avoids those risks.
It’s long been known that mutations in the retinoblastoma tumor suppressor gene RB1 are behind almost all cases of hereditary retinoblastoma (and many sporadic cases too) – but no treatment to date has exploited this knowledge. Bearing this in mind, a team led by Rajesh Rao from the University of Michigan in Ann Arbor reasoned that since RB1 is upstream of the epigenetic regulator EZH2 (which is also a pharmacologic target for many solid tumor therapies in development), EZH2 might represent a good target for future retinoblastoma therapies (1).
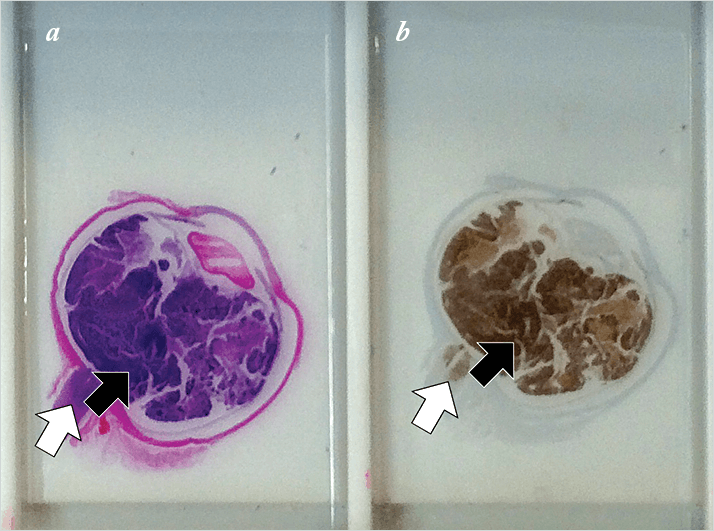
Using immunohistochemistry, Rao’s team showed that the EZH2 protein was enriched in the retinae of human fetuses and was not expressed in the normal postnatal retina (Figure 1). However, in human retinoblastoma enucleation samples, EZH2 was present, enriched, and delineated the extent of the entire intraocular tumor. Further, the team discovered that that they could resolve the metastasis of a single EZH2-expressing tumor cell into adjacent tissues, such as the inner plexiform layer and the optic nerve – a key factor in the decision to choose systemic chemotherapy over local, ocular interventions. The team investigated the role of EZH2 in retinoblastoma cell survival by treating human retinoblastoma and primary retinal pigment epithelium (RPE) cells with inhibitors of EZH2 – either GSK126 or SAH-EZH2. The highest concentration of GSK126 inhibited EZH2 expression, and both drugs managed to impair intracellular adenosine triphosphate (ATP) production (an indicator of cell viability) in retinoblastoma cells in a time- and dose-dependent manner. Crucially, neither EZH2 inhibitor showed any effect on primary human fetal RPE cell ATP production – possibly because EZH2 expression in this tissue is far lower than in retinoblastoma tumor cells. According to the authors, this study marks the first time that this mechanism has been implicated for an ophthalmic, adnexal, or orbital tumor they believe that the specificity of EZH2 inhibitors toward human retinoblastoma tumor cells – but not RPE – warrants further in vivo testing in animal models of retinoblastoma, particularly those EZH2 inhibitors currently in clinical trials for solid tumors and lymphoma.
References
- M Khan, et al., “Characterization and pharmacologic targeting of EZH2, a fetal retinal protein and epigenetic regulator, in human retinoblastoma”, Lab Invest., [Epub ahead of print] (2015). PMID: 26280220.
