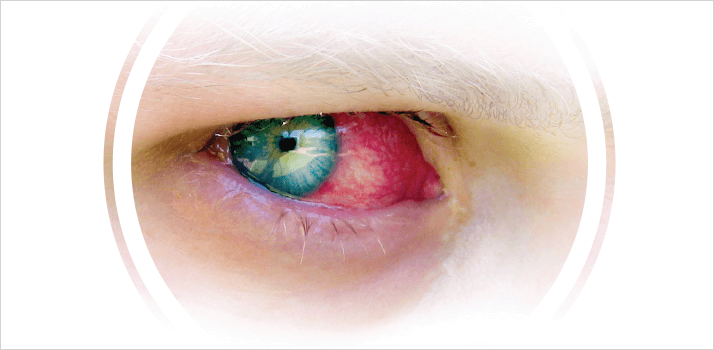
Itching. Irritation. Dry eye. In extreme cases, keratosis and scarring. Adverse events occur in one in 10 patients who are on long-term therapy with eyedrops for conditions like glaucoma or uveitis. For many, these side-effects are caused by preservatives.
Why do eyedrops have preservatives?
In essence, to stop bacteria growing. Patients with chronic eye diseases tend to be elderly and have poor sight and reduced dexterity; they can have difficulties using (and reusing) eyedrops in a way that minimizes the chance of bacterial transfer. And bacterial infection is something to be avoided: conjunctivitis is never pleasant, and in cases where the eye isn’t intact, like in corneal abrasion, infections that penetrate into the eye can be serious. Hence the preservatives.What is used and how does it work?
Benzalkonium chloride (BAK) is the preservative of choice; mercurials, chlorhexidine and chlorobutanol have also been used. BAK is a surfactant; at the concentrations used in eyedrops, it acts as a bactericide, dissolving bacterial cell membranes. BAK also has cleaning properties, and is used at higher concentrations as a detergent in many soaps, cosmetics, disinfectants and spermicides.What are the potential side-effects of BAK?
BAK not only irritates the eye, it can also damage ocular structures (1). Often patients use more than one BAK-containing eyedrop and, as Russell Young of the International Glaucoma Association explains: “The allergic response to BAK is dose-related. Anyone on two or three medications has an increased exposure to BAK, increasing the likelihood of strong irritation or an allergic response” (1). This has been mitigated in recent years by the introduction of combined drops. “It has been very useful,” notes Young, “for example, with Cosopt you've got timolol and dorzolomide in the same bottle, reducing the patient’s exposure to BAK.”Can BAK be eliminated altogether?
Yes, preservative-free preparations of some of the most commonly-used glaucoma medications (including timolol, pilocarpine, travoprost, tafluprost, bimatoprost, latanoprost and even Cosopt) are either now available, or will be shortly. To minimize bacterial contamination risk, they come in single-use, disposable packaging. This raises some issues – they are small and awkward to use. Despite the packaging containing more than double the volume required for a single use, there is the possibility that none of it will be successfully applied. “A small number of patients have difficulty using the unit dose preservative-free drops – and compliance aids are not yet available to assist them,” Young says.So why is BAK still used?
It comes down, partly, to cost. BAK-containing eyedrops cost less than preservative-free eyedrops. Some of the BAK-associated adverse events, like dry eye, can be controlled with different eyedrops, although this also increases the cost. With healthcare systems around the world under pressure to cut costs, and with 90 percent of patients tolerating BAK, “giving preservative-free eyedrops for all patients is difficult to justify,” states Young. On the other hand, those patients who have poor outcomes increase the cost to the healthcare provider. “Every patient must be considered as an individual and treated appropriately,” is Young’s conclusion.Is BAK always to blame?
No, patients may be sensitive to other eyedrop components. “If you’ve got a patient on multiple drops and they’re starting to develop allergic reactions, it’s difficult to decide if it is the BAK exposure, or one of the compounds from the three drops they’re taking,” says Young. “The ophthalmologist might try replacing BAK-containing eyedrops with preservative-free eyedrops – if these are available, and if appropriate – or go down the laser surgery or trabeculectomy route to reduce exposure to the irritant. It’s a judgment call for the ophthalmologist.” ?References
- J. Hong and L. Bielory “Allergy to Ophthalmic Preservatives”, Curr. Opin. Allergy Clin. Immunol., 9 (5), 447–453 (2009).
