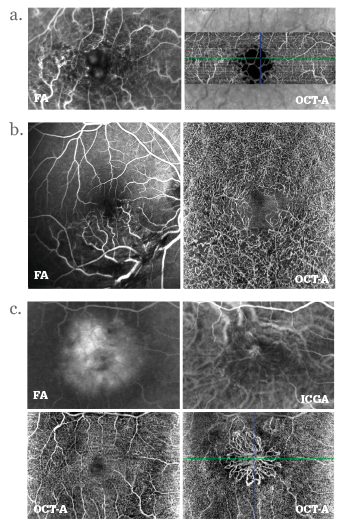
• OCT angiography (OCT-A) can provide clear, depth-resolved visualizations of the retinal and choroidal microvasculature, unobscured by the staining or pooling effects seen in FA and potentially offers a rapid, high-quality and low-risk alternative to fluorescence angiography
• Some limitations exist, like the inability to visualize leakages, but OCT-A holds great diagnostic potential in many clinical situations, once clinical validation is completed
• Obtaining the best possible OCT-A requires high-quality OCT volume images that can be produced reliably and reproducibly, and involves the suppression of eye motion artefacts like micro- and macro-saccades
• Active eye tracking technology – such as that present on the SPECTRALIS diagnostic imaging platform – helps avoid motion artefacts during OCT volume scan acquisition
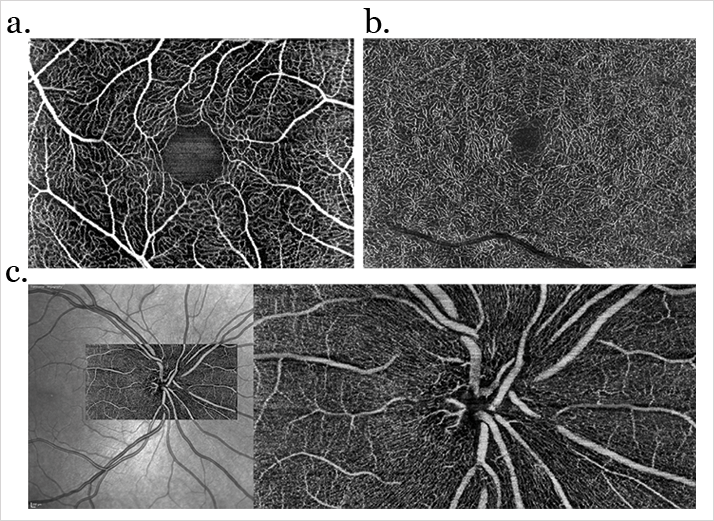
Many posterior segment ocular diseases involve the retinal and choroidal vasculature. From neovascular age-related macular degeneration, diabetic macular edema to geographic atrophy, the vasculature of the retina changes as the disease progresses. Fluorescence angiography – typically using fluorescein (FA) or indocyanine green (ICG) as the fluorescent contrast agent – has been the standard method of its assessment, but both have a number of problems associated with their use. The systemic intravenous injection of fluorescein or ICG can result in systemic adverse events like nausea and allergic reactions (occasionally anaphylaxis), and it consumes both time and resources: patients have to be prepared before the procedure, monitored afterwards, and the contrast agent isn’t free of charge. There’s a compelling, non-invasive new method to examine the eye’s vasculature emerging: optical coherence tomography (OCT) angiography (OCT-A). Described by Gabriel Coscas, Emeritus Professor of the Hôpital Intercommunal de Créteil’s Ophthalmology Department, as “a revolutionary technique”, OCT-A can provide a “clear, depth-resolved visualization of the retinal and choroidal microvasculature” (Figures 1 and 2). The Chairman and Professor of the University of Bonn’s Department of Ophthalmology, Frank Holz, is similarly enthusiastic, believing that “OCT-A represents a fascinating new technology to noninvasively visualize vascular pathology associated with a wide spectrum of macular and retinal as well as choroidal diseases.”
Ophthalmologists’ views on the potential impact of OCT-A
OCT-A is a relatively new technology, but it carries the potential to significantly change the routine assessment and characterization of certain retinal and choroidal diseases. “Today, most OCT-A devices are still at the prototype stage, and we are still trying to understand their full potential,” noted Coscas, “but it looks like that OCT-A could be an extremely useful addition to traditional multimodal imaging for the routine evaluation of patients with retinal or choroidal disorders.” He added: “Despite the great diagnostic potential, as the physical principle is to measure moving objects (such as erythrocytes), the technology is not able to capture certain clinical findings such as leakages or pooling of the dye, or to differentiate between arteries and veins, or observe slow vascular flow in micro-aneurisms. Clearly, this means that OCT-A will need to be used in conjunction with traditional FA in some diseases, but in others, we are confident that OCT-A has the potential to replace FA. However, before we make any definitive statements, we need a more thorough understanding of OCT-A technology, with all its advantages and limitations, and an extensive clinical evaluation.” Holz addressed the issues of cost and comfort: “Noninvasive OCT angiography takes much less time compared with invasive angiography with fluorescence dyes, and is more comfortable for the patient. The resolution of the recorded images is usually excellent – the perfused retinal and choroidal vasculature is readily visualized and (unlike FA), is unobscured by staining or pooling effects. Overall, the procedure is less costly than FA, given that there is no need for a nurse or a physician to perform the fluorescein bolus injection,” adding, “There is of course the cost for the instrument, but given that spectral domain (SD)-OCT is widely used for the evaluation of retinal diseases, most retinal specialists usually have one anyway.” Some existing OCT devices can even be upgraded to OCT-A, further reducing the investment costs involved. “The speed of the procedure is not the main issue, as it depends not on the acquisition, but the time spent by the clinician to obtain a correct diagnosis,” added Coscas, “and as OCT-A can easily be performed each time the patient is evaluated – without dilation of the pupil – this makes for a very significant advantage over FA.” Holz further explored the diagnostic potential of OCT-A, going on to explain that he “sees great potential in early detection of disease, for example, by visualizing both the inner as well the deep retinal vascular plexus, which may show pathology early on in disease processes such as macular telangiectasia type 2,” adding, “This is a nascent technology, and it still requires further validation, but I see enormous research potential for OCT-A. Besides giving us a deeper understanding of retinal and choroidal diseases, it opens up new possibilities for assessing longitudinal vascular changes both in natural history and interventional studies – for example, giving us a better understanding of therapeutic effects of repeated doses of anti-VEGF drugs.” In terms of clinical trials, Coscas can also see the benefits: “Angiographic examinations are usually performed at the initial evaluation and at pre-established checkpoints, generally far from clinical trial enrollment. As OCT-A is both fast and noninvasive, it could be repeated on each follow-up visit, and therefore provide a more detailed quantification of the evolution of the disease and of the efficacy of treatment than has been possible to date.” In other words, OCT-A might help us better understand the effects of the current generation of anti-VEGF agents and steroids on the retinal and choroidal vasculature, and also more comprehensively evaluate the effects of the next generation of such drugs in a clinical trial setting.The technical challenges involved in producing good OCT-A images
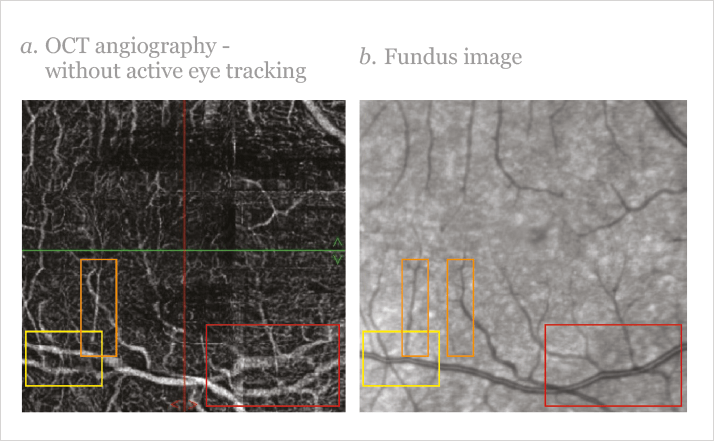
“OCT-A works by measuring the movement of light-scattering objects in a system – in a static eye, the only moving structure should be blood flowing through the vasculature,” explained Coscas, noting that “by calculating the changes in the signal amplitude from repeated consecutive B-scans at the same section, a motion contrast between static and non-static tissue is generated, enabling visualization of three-dimensional retinal and choroidal vasculature and microvasculature.” In order to obtain a good OCT-A reconstruction of the retinal or choroidal vasculature, you need a high-resolution, very dense, three-dimensional OCT volume dataset. To achieve that, you have to start with lots of high-quality B-scan images – but this takes time. Even high-speed OCT instruments, which can perform 85,000 A-scans per second or more, take several seconds to acquire the dense three-dimensional OCT volume dataset required for OCT-A. Coscas explained that the density of the final dataset isn’t the only key to a successful scan – “Bulk motion – any movement of tissue with respect to the OCT device, be it head or eye movements – has to be considered, and has to be sufficiently compensated for, as blood circulation needs to be the predominant source of temporal changes between OCT scans – inadequate compensation makes for inaccurate scans.” In order to be completely effective, OCT-A technology must be able to account for both small-scale (micro- and macro-saccades) and large-scale (conscious movement) changes in the position of the eye. “The consequences of bulk motions could be partially avoided with a very high acquisition speed, but if you consider that eye movements, especially microsaccades, have a very high likelihood of happening even during a very fast scan, something more is needed,” added Coscas, noting that “an active eye tracking system, such as one based on the principle of simultaneous acquisition of fundus images and OCT images, may sufficiently mitigate eye motion artefact, enabling unblurred images to be obtained. An active eye-tracking system (Figure 3) presents a very reliable method to acquire OCT volume scans without motion artefacts because eye motion is identified and accounted for during data acquisition. Only images free of motion artefacts are being saved.”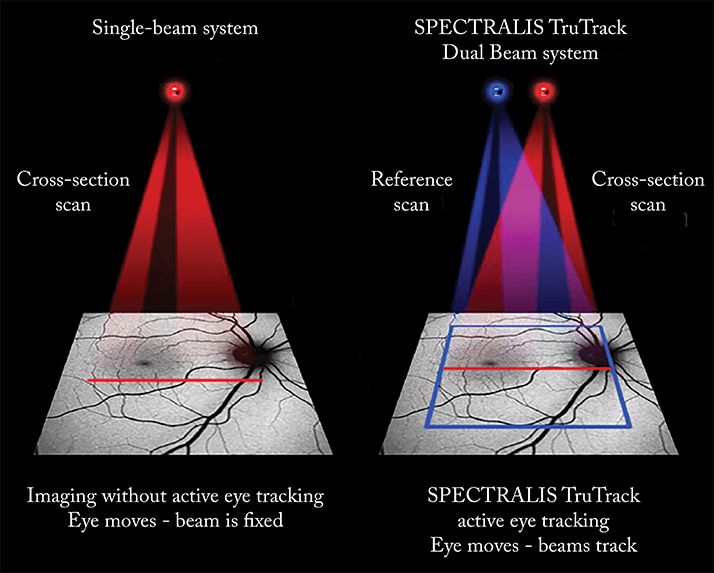
The importance of eye motion correction should not be underestimated. Without it, the diagnostic capabilities of the instrument become severely limited – or in the worst case, create artefacts that cannot be unambiguously separated from retinal abnormalities (Figure 4). One alternative method of correcting for bulk movements – post-acquisition motion correction – tries to eliminate lateral movements by acquiring one volume scan horizontally and one vertically. However, diagnostically relevant information that has been missed due to eye motion during the exam cannot be retrieved at a later time. In contrast to an active eye tracking system, the reconstructed image might miss information from certain anatomic locations of the retina. Finally, in order to follow disease progression or the effect of a therapy in a clinical trial at different time points, it’s important to be able to perform OCT-A at different times – in precisely the same retinal location.