ILUVIEN® is an intravitreally-administered fluocinolone acetonide implant that, once administered, delivers therapeutic corticosteroid concentrations for a 36-month period. The National Institute for Health and Care Excellence (NICE), the organization that decides which drugs and treatments are available on the UK National Health Service (NHS), recently approved ILUVIEN® as an option for treating chronic diabetic macular edema (DME) that is insufficiently responsive to available therapies (only if the implant is to be used in an eye with an intraocular [pseudophakic] lens and the manufacturer provides fluocinolone acetonide intravitreal implant with the discount agreed in the patient access scheme [1]). This means that ILUVIEN® will soon be available for use in clinical practices across the UK. “Available therapies” include ranibizumab for patients with DME and a central foveal thickness (CFT) greater than or equal to 400 µm, and laser photocoagulation for those with a CFT <400 µm. However, NICE’s approval wording does not define what either “chronic DME” or “insufficiently responsive to available therapies” actually means. To address these issues, Alimera Sciences organized an expert roundtable meeting on September 27, 2013 at the Marriott Courtyard Hotel, Hamburg, during the XIIIth EURETINA congress. This article summarizes that event.
Roundtable participants
Chair: Yit Yang1Expert panel members: PeterAddison2 Martin Harris9
Frank Ahfat3 Simon Horgan2,10
Tariq Aslam4 Roland Ling11
Somnath Banerjee5 Luke Membrey3
Mandeep Bindra6 Arijit Mehta12
Helen Cook7 Tree Richardson13
Louise Downey7 Maria Teresa Sandinha14
Haralabos Eleftheriadis8 Sandy Taylor†15 Plus representation from Alimera Sciences & Visions4Health *All are UK-based Consultant Ophthalmologists/ Consultant Ophthalmic Surgeons, unless indicated; †Nurse Manager/AMD Research Study Coordinator.
1 Royal Wolverhampton Hospital; 2 Moorfields Eye Hospital, London; 3 Maidstone Hospital, Kent; 4 Manchester Royal Eye Hospital; 5 University Hospitals of Leicester NHS Trust; 6 Stoke Mandeville Hospital, Aylesbury; 7 Hull and East Yorkshire Eye Hospital; 8 King’s College Hospital, London; 9 The Royal Free Hospital, London; 10 St George’s Hospital, Tooting; 11 Royal Devon & Exeter Hospital; 12 Birmingham & Midland Eye Centre; 13 Croydon University Hospital; 14 Sunderland Eye Infirmary; 15 St. Paul’s Eye Research Centre, Liverpool.
Defining chronic DME
The discussions were led by Professor Yit Yang, Consultant Ophthalmic Surgeon at the Royal Wolverhampton Hospital. “To tackle chronic DME, you need to understand why NICE used that term,” he explained, “and so we go back to the Fluocinolone Acetonide in Diabetic Macular Edema (FAME) trial (sidebar below) and examine why a median time period of three years was used to define chronic DME”.ILUVIEN®’s UK indication is based on data from the FAME trial
- FAME consisted of two parallel trials with identical designs that compared two ILUVIEN® doses with “best standard of care” for the treatment of DME (4)
- The trials were presented together as a merged dataset comprising data from 953 patients with CFT greater than or equal to 250 µm
- The primary efficacy endpoint was the difference between the treatment and control groups in the percentage of patients whose best corrected visual acuity (BCVA) improved by greater than or equal to 15 letters at month 24 compared with baseline levels
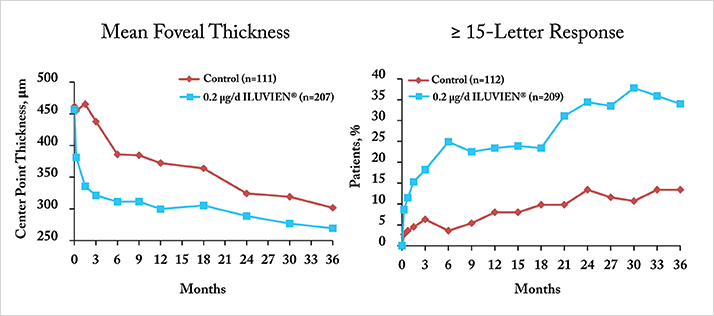
- ILUVIEN® was significantly more efficacious at 24 and 36 months than than the best standard of care in patients with chronic DME (Figure 1)
Before ILUVIEN®: the 400 µm ranibizumab DME divide
- Before 2011, the standard of care for treating DME-associated visual impairment was laser photocoagulation therapy
- In 2011, NICE approved ranibizumab for the treatment of DME-induced visual impairment – but only for patients with CFT greater than or equal to 400 µm at the start of treatment (2)
- This generated two DME patient populations, those that can receive ranibizumab, and those that cannot
- The ranibizumab summary of product characteristics states that if no visual acuity improvements are seen after “the first three injections, continued treatment is not recommended” (3)
“Chronic” was defined by the median DME duration among patients at enrolment into the FAME trial; this was found to be three years. Philip Ashman of Alimera Sciences noted that a conservative method was used to calculate the median duration of DME (sidebar above); as one year was added to all calculated durations of DME. “Why plus one? Some patients have only the year of diagnosis recorded, not the exact date,” he explained. “So the median of three years is only according to this strict algorithm. If we take actual dates, and pick midpoints of years where only the year is available, and look at the median again, it’s 1.73 years. This needs to be clear to CCG (Clinical Commissioning Group) commissioners” (Figure 2).
On the basis of the strict algorithm definition of three years for chronic DME, Yit asked: “So if you have a patient with chronic DME, they had their first laser nine months ago, their CFT is already >400 µm, and you’re about to start ranibizumab, Are you happy to have to wait three years before you can use ILUVIEN®?” The absolute consensus was no. Tariq Aslam pointed out that the situation facing clinicians today is a “different ballgame” to 2007 when the first patients were recruited into FAME. “For that population laser therapy was three years away. Now, they may have had multiple ranibizumab injections and still shows edema. There is a clear rationale for reducing the time period from three years.” Peter Addison agreed – to general consensus – “there is no reason to use that definition of three years”, and added, “I think we need to clarify the term ‘chronic edema’ – are we thinking of the term timespan, or are we talking about anatomical change?”
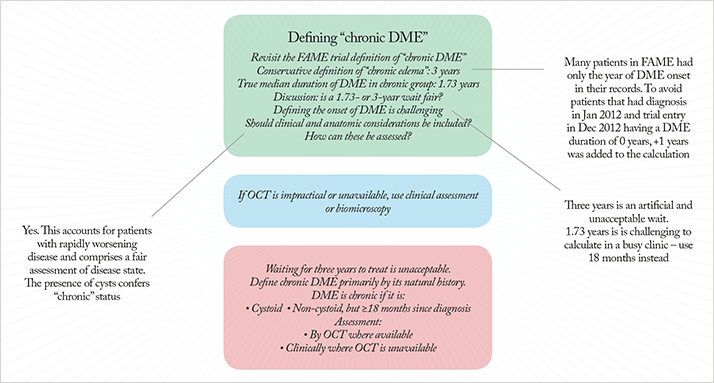
To explore Peter Addison’s question, Yit reviewed the natural history of the morphology of DME. “It starts with a spongiform morphology, which later becomes cystoid,” he noted. “Can edema be termed ‘chronic’ when it is so bad that the fluid becomes extracellular – meaning that the swelling is destroying cells?” he asked, adding, “cystoid morphology is evaluable with optical coherence tomography (OCT).” This concept was well received. Simon Horgan expressed his enthusiasm for an anatomical definition of chronic DME. “It gives the capacity to cope with a change in the rate of DME. We all know that it can change at a disproportionate rate. For instance, we can have a very innocuous-looking DME, then the patient has a cataract removed, and the DME accelerates; the macula thickens, and you’re straight away into a chronic DME picture that we would recognize.” This prompted Yit to state, “So you can have a patient that you’ve just operated on, they’ve gone cystoid, but they’ve only had DME for one year,” leading him to ask the expert panel, “Are the 1.73-year and the three-year cutoffs ridiculous in terms of clinical management of the patient?” Simon Horgan said that these time limits, “made things artificial”. Tree Richardson took a more pragmatic approach: “You could just add ‘or’ to the chronic DME definition – cystoid morphology or 1.73 years,” before suggesting that 18 months be used instead of 1.73 years – for ease of calculation. She noted that although OCT confirmation of cystoid morphology was desirable, some patients may be unable to undergo OCT due to mobility or morbidity reasons. A short discussion between panel members led to agreement that clinical assessment or biomicroscopy can be used if OCT was unavailable. Yit summarized the consensus (Figure 2):
- Waiting for three, or even 1.73 years to treat chronic DME is unacceptable
- Chronic DME should be defined primarily by its natural history, then by duration: if it is cystoid, it is chronic; if it is non-cystoid, it needs to have been present for over 18 months to be considered chronic
- Assessment should be made by OCT where available, but clinical assessment is acceptable where OCT is unavailable
Defining “insufficiently responsive”
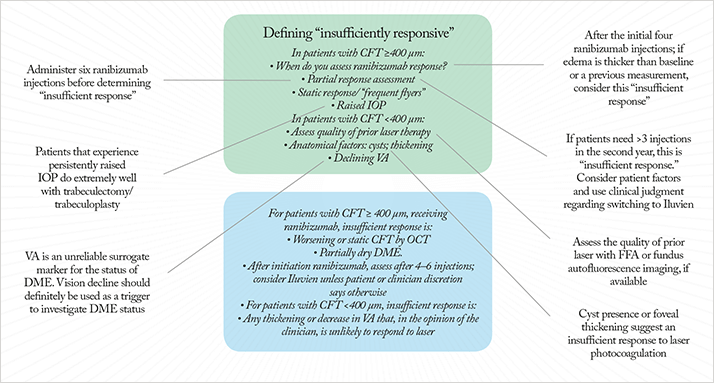
In patients that can receive ranibizumab “In the current DME pathway, we have patients with CFT >400 µm; patients who, according to current guidelines, we are supposed to treat with intravitreal ranibizumab,” noted Yit. “The number of ranibizumab injections will vary from person to person, but what we don’t know is: what does ‘insufficiently responsive to ranibizumab’ means in these patients?” To Yit’s question, “How many injections would you go for before deciding on insufficient response? When do you start looking at the response?” Roland Ling replied that, “In practice you give the patient three or four injections as a loading dose. So within four. That’s when I would do the OCT comparison with baseline CFT measurements.” Yit posed the questions: “If OCT showed that edema was worse after the fourth ranibizumab injection than at baseline (or after earlier injections), is that ‘insufficient response’?” The panel agreed. Yit asked the panel if they thought that using visual improvement to assess treatment response was a valid method in this patient group. The response was unanimous: “Vision is unreliable”. Nevertheless, Yit noted that he was “happy to use declining vision as a trigger to investigate whether the patent is insufficiently responsive to ranibizumab.”Mandeep Bindra posed a question about patients that have improved with ranibizumab, but aren’t completely dry. Martin Harris advised that in these cases, “You wait until six injections – only after then you can say you have insufficient response.” Mandeep then discussed “frequent flyers” – patients that need to continue monthly ranibizumab injections, as dropping bi-monthly injections results in vision degradation. Yit suggested, “If patients required more injections than in the DRCR.net protocol, that is, more than three in the second year of treatment, then that should also be considered ‘insufficient response’.” Arijit Mehta felt that “we should give patients the choice at that point”, which Yit acknowledged, modifying the recommendation to: “Static, but greater than three injections, in the second year; consider patient factors.”. Simon Harris made a popular observation: “Isn’t that micromanaging things?” His thoughts were that “there needs to be a bit more discretion with the treating ophthalmologist. I think we’re perfectly bang to rights to say: a measurable clinical response. And that can be interpreted by the treating physician.” This was backed up by Louise Downey, who described patients that have achieved only partial response with ranibizumab – but who were very happy with their improvement in vision, who you would “have a hard time convincing to switch therapies”. Accordingly, Yit suggested that the pathway be: “after the four-to-six injections, convert to ILUVIEN®, unless your clinician's discretion comes up with some reason not to switch.”
In patients that can’t receive ranibizumab The panel then turned to what constitutes “insufficient response” in patients with CFT <400 µm. Such patients have already undergone laser photocoagulation therapy, but their CFT is too thin to receive ranibizumab. Yit asked: “What do you do? How do you define insufficiently responsive in these patients?” Simon Horgan replied that, “The first thing I’d do is make sure they’ve had adequate laser therapy,” observing that, “Just because someone’s documented that they’ve had macular laser, it doesn’t mean that it has been done well.” He said that it was a matter of “performing fluorescein angiography and fundus imaging to assess that.” This immediately raised the question: should another laser treatment be performed? Louise Downey stated that she thought that “OCT is more important in this category, as the visual acuity response is such a slow effect,” leading Yit to summarize the following options:
- A CFT cutoff without it being cystoid or non-cystoid. For example, a CFT >300 µm would define “insufficient response” to laser.
- Apply at OCT/CFT cutoff.
Simon Horgan interjected: “Why does it have to be one?” which resulted in Yit changing the statement to “Cystoid, or thickening above 300 µm”. Helen Cook asked: “Why can’t you have thickening that’s not improved or is worsening, despite laser? Rather than having to put a number on it.” This led to Yit proposing a final scenario for patients with CFT <400 µm on which consensus was finally achieved: “We have a patient who has had five lasers over the past few years; their CFT is still under 400 µm, and they still have thickened macula – we may see spongiform or cystoid thickening, and you say that they have reduced visual acuity – 6/18 or 6/12, say – purely due to edema, not cataract or anything else. In your opinion, further laser is not going to help. You eyeball that patient and you say, look, they’ve got laser scars there, they haven’t got any leaking micro-aneurysms that you can see, let’s go for that definition for an ‘insufficient response’ to laser” (Figure 3).
Conclusion
The roundtable meeting addressed some issues of substantial importance. ILUVIEN® has the potential to preserve and improve the vision in many patients with chronic DME – patients whom NICE described as being “insufficiently responsive to available therapies”. The expert panel achieved consensus on what “chronic DME” actually meant, by examining the clinical trial data that underpinned NICE’s decision to approve ILUVIEN® for that indication, and bringing to it their front-line clinical experience of managing these patients today, determining that anatomical – not chronological – is the best place to start when diagnosing chronic DME. NICE did not describe what was meant by “insufficient response to available therapies” in their approval, which meant that the interpretation of that statement might vary. The expert panel formed consensus guidance on what they believe “insufficient response” to mean, based not only on careful study of the clinical trial data that supported NICE’s approval of ranibizumab for treating DME in patients with CFT greater than or equal to 400 µm, but also based on their experience of treating patients with ranibizumab, including important patient types such as partial ranibizumab responders, and “frequent flyers.” Furthermore, for patients with chronic DME and a CFT <400 µm, they established an algorithm for defining who these patients were, what was meant by insufficient response to laser therapy, and when ILUVIEN® should be administered.References
- National Institute for Health and Clinical Excellence. Final appraisal determination. Fluocinolone acetonide intravitreal implant for the treatment of chronic diabetic macular edema after an inadequate response to prior therapy (2013). http://www.nice.org.uk/nicemedia/live/13304/61736/61736.pdf National Institute for Health and Clinical Excellence. Ranibizumab for treating diabetic macular edema. Rapid review of technology appraisal guidance 237, (2011). http://www.nice.org.uk/nicemedia/live/13125/55324/55324.pdf Lucentis Summary of Product Characteristics (2013). http://www.medicines.org.uk/emc/medicine/19409. PA Campochiaro, DM Brown, A Pearson et al., “Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema”, Ophthalmology, 119, 2125–2132 (2012).
