The European Society of Cataract and Refractive Surgeons (ESCRS) 2021 Congress hosted a symposium for ophthalmic surgeons titled “ANTERION – Better Solutions by Accuracy. Anterior Segment Diagnostics and Biometry,” featuring four distinguished experts: Oliver Findl, Kjell Gunnar Gundersen, Bjørn Gjerdrum, and Damien Gatinel, who presented results from their clinics. The meeting was chaired by Findl, who thanked the organizers, Heidelberg Engineering, and graciously welcomed the audience gathered in the room – many of whom were attending their first in-person ophthalmic event since the start of the COVID-19 pandemic – and those in front of computer screens, connecting virtually.
Findl’s opening presentation began with an overview of Heidelberg Engineering’s ANTERION – a multimodal imaging platform optimized for a wide range of applications in the anterior segment, with functionalities, or “apps,” such as the Cornea App, the Cataract App, the Metrics App, and the Imaging App. As Findl explained, the platform boasts a wavelength of 1300 nm, a scan rate of 50,000 Hz, axial resolution of 10 microns, and it produces B-scans with the length of 16.5 mm length and depth of 14 mm, meaning that the entire anterior segment is displayed within the image frame. It also provides A-scans for the axial eye length (AL), required for biometry purposes. Corneal topography of the platform takes 65 B-scans with 9 mm length. Corneal tomography is also offered; it’s important to remember that topography and tomography differ in excluding or including the posterior corneal surface, respectively, and tomography is ANTERION’s exceptional strength. The AL measurement automatically detects the retinal pigment epithelial peak – the most relevant parameter for measuring of AL.
Findl then presented the results of the Agreement Study, which compared two SS-OCT biometry devices: the IOL Master 700 (Carl Zeiss Meditec AG) and the ANTERION (1). Comparable metrics assessed included: AL, anterior chamber depth (ACD), lens thickness (LT), and keratometry. 389 eyes of 209 patients with age-related cataracts were measured using both devices and found very similar measurements of AL, keratometry, ACD, and LT, with very few outliers. As Findl noted, any differences were found not to be clinically relevant when performing an IOL power calculation.
“The mean absolute difference between the keratometry data of the two devices was 0.04 ± 0.05 mm (7.80 ± 0.26 mm for biometer A and 7.82 ± 0.26 mm for biometer B; P < .0001) for the steep keratometry readings and 0.04 ± 0.04 mm (7.63 ± 0.26 mm and 7.65 ± 0.25 mm; P < .0001) for the flat keratometry readings. For ACD and LT, the mean absolute difference was 0.07 ± 0.04 mm and 0.07 ± 0.04 mm. The mean absolute difference for AL was 0.02 ± 0.03 mm (23.55 ± 1.18 mm for biometer A and 23.54 ± 1.18 mm for biometer B; P < .0001).” (1)
Findl then went on to share results of another study, designed to determine repeatability. The study used the same two SS-OCT biometers, as well as the OLCR Lensar LS900 (Haag-Streit AG) and measured 50 eyes of 50 patients. It found that all the devices included presented high repeatability, with the two SS-OCT devices outperforming the OLCR device, which showed a larger number of outliers (2).
The study also assessed pre- and post-op AL, measuring 50 eyes of 50 patients suffering from cataracts, using the IOL Master 700 and the ANTERION. Based on the evaluation of pre-and post-op changes to AL measurements, it turned out that the difference for both devices (0.08 mm for the IOL Master 700 and 0.07 mm for the ANTERION) was statistically significant, but not clinically relevant. A small correlation between cataract grades and AL measurement difference was found.
The final comparison presented by Findl looked at higher order aberrations (HOAs) in patients scheduled for cataract surgery using an SD-OCT with Placido system (CSO, MS-39) with the ANTERION. It found very little to no difference in HOAs. As Findl concluded, the ANTERION showed very high reproducibility of results, with measurements similar to those of the IOL Master 700.

Gundersen began by reminding the audience that in modern refractive surgery, obtaining accurate biometry measurements is mandatory in all cases – normal and irregular. He went on to show the results of a pilot study that examined refractive results 5-6 weeks post-op in 41 eyes of 21 patients. Gundersen and colleagues found that the ANTERION returned the lowest predictive error – both arithmetic and absolute – and a clear trend was visible even within the small cohort.
Gundersen commented on the epidemiology of astigmatism and noted that with 45 percent of patients presenting with corneal astigmatism over 1 D, every second IOL implanted should or could be a toric IOL. His clinic conducted a study looking at low toric (T2) cylinder lenses implanted, and found that post-op refractive cylinder and the uncorrected contrast sensitivity were significantly better when toric lenses were used. Gundersen then went on to present special cases in which ANTERION had proven particularly useful.
Case 1
● The right eye of a 62-year-old male patient with post-LASIK keratitis
● Laser regularization and scarring
● Asymmetric astigmatism axis and power
● Pre-laser vision correction (LVC) refraction: -+5.00-1.75x45, VA 1.0
● Pre-cataract refraction: -+7.00-1.50x15, VA 0.8
● Autorefractive: +10-2.0x55
● Preoperative clinical challenges: anisometropia, reflections, and headache
A thorough biometry work-up was done, arriving at a “conservative” IOL choice. When the predictive spherical and cylindrical errors were checked, the sphere was close to perfect, but the cylinder was still a challenge. In week 5 post-op, the patient’s VA was 0.8, with fewer side effects and anisometropia.
Case 2
● A male patient of 63 years with stable keratoconus
● The central part of his left eye more affected by keratoconus than the center of this right eye:
OD: 41.85, astigmatism 5.98 @137, AL 24.82
OS: 46.32, astigmatism 9.61 @39, AL 24.60
● Stable refraction pre-cataract
● Pre-cataract refraction
OD: -4.00-2.50x455 VA 0.7
OS: -1.25-0.50x45 VA 0.6
Multiple biometry measurements were done, and IOLs were implanted for a large astigmatism correction (OD: +15.00 D, cylinder +7.50 D, OS: +14.00 D, cylinder +10.00 D). Following the surgery, the right eye turned out to be slightly undercorrected, and the left eye was slightly overcorrected. The spherical component was – again – close to perfect, but the toric component posed more difficulty. Five weeks post-op, the patient “had never seen better,” he was uncorrected in bright light, reading well with a simple “plus” lens.
Gundersen summarized his findings from irregular cases, pointing out that patients’ expectations have to be realistic, corneal surface needs to be optimized, thorough biometry should be performed, and access to a wide range of toric IOLs is very important. All this should be accompanied by the surgeon’s wealth of experience dealing with similar cases.
Gundersen has found ANTERION to be the only instrument that can acquire all measurements for ray tracing in one setting, which results in fewer measurement errors. In his experience, it’s ready for any modern, fifth generation biometric formula, useful in an increasing number of cases, as well as clinically and scientifically reliable. He can see even more potential for integrating ANTERION with imaging systems; image averaging of a minimum of three images could reduce the variability; access to epithelial maps, and objective dry eye assessment.
Gjerdrum opened with a comment on the challenge of obtaining accurate post-LVC calculation, which often makes traditional formulas unusable, due to keratometric index errors, ELP errors, and radius/instrument errors. Therefore, special post-LVC formulas have been developed, taking into account historic, pre-LVC data, or no-history formulas based on regressions to predict true corneal power, using assumed posterior or total corneal power. They are all theoretical formulas that use paraxial assumptions, which are not accurate for the human eye.
Ray tracing IOL calculations, on the other hand, are exact calculations based on Snell’s law, using single rays going through each refractive surface at varying radial distances. These calculations use no paraxial assumptions, but the accuracy is dependent on the available input data: the amount, and the accuracy of it.
Gjerdrum presented results of a study comparing the refractive precision of ray tracing IOL calculations (done using the ANTERION and Tomey Casia SS-1000) with post-LVC formulas (done using Haag-Streit Lenstar 900) in 37 eyes of 20 patients who had undergone LVC for myopia (3). 65 percent of patients had toric IOLs implanted. Patients were examined two to three months after surgery, and the main outcome metric – refractive prediction error – was measured as the post-op refraction minus the predicted refraction. The study found that ray tracing based on the ANTERION platform OCT data gave results similar or better than those based on post-LVC formulas. ANTERION in combination with OKULIX (a ray tracing calculation software integrated on the ANTERION platform) had the best arithmetic mean RPE, lowest “range,” and 60 percent of the results were within ±0.25 D. Gjerdrum noted that this was also the only calculation that had all eyes within three quarters of a diopter. He concluded pointing out that the main advantage of ray tracing is that it takes individual measurements and is independent of ocular history, so it should be suitable for any eyes.
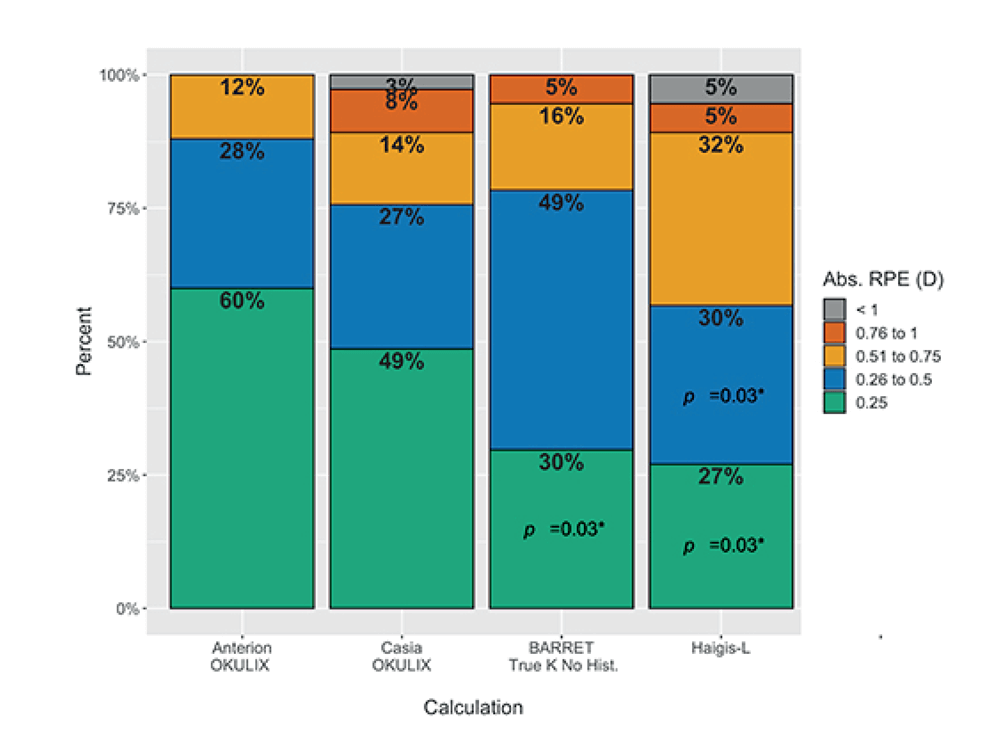
“The best ray tracing combination resulted in an arithmetic prediction error statistically significantly lower than that achieved with the best formula calculation (Barret True-K, no-history) (-0.13 D and -0.32 D, respectively, adjusted p = 0.01), while the Barret TK NH had the lowest SD. The absolute prediction error was 0.26 D and 0.35 D for ANTERION-OKULIX and Barret TK NH, respectively, but this was not statistically significantly different. The ANTERION-OKULIX calculation also had the highest percentage of eyes within ±0.25, compared to both formulas and within ±0.50 and ±0.75 compared to the Haigis-L (p = 0.03).” (3)
Gatinel, presenting the work he did with Alain Saad from the Anterior Segment and Refractive Surgery Department, Rothschild Foundation, Paris, France, began by reminding the audience that post-LASIK keratectasia is one of the most devastating complications of LASIK, and a bugbear of all refractive surgeons, despite its low incidence. Even though many approaches are available, surgeons are still trying to improve their detection methods. The “comfort level” or “threshold” for LASIK varies from one surgeon to another, and can be subjective. “Therefore,” he said, “a simple, efficient, yet objective risk assessment system is required.”
Gatinel illustrated subjectivity in the field by presenting a study that aimed to evaluate the variability of experts’ subjective corneal topography map classification (4). Eleven corneal topography experts were asked to rank corneas according to the Ectasia Risk Scoring System depending on preoperative axial curvature maps using Scheimpflug imaging obtained with the Pentacam HR (Oculus) and clinical parameters. The study found significant variability among experts in subjective classifications within the same scale, and significant variability for each expert when working with different scales.
Using an example of two eyes of a keratoconus patient, and an observation by Stephen D. Klyce, Gatinel notes that where one eye is affected, the other eye – which has no clinical findings except for some topographical changes, should be considered forme fruste. Gatinel sees those eyes as good subjects for a training system to detect abnormalities, and commented that they should be monitored until they become suspect.
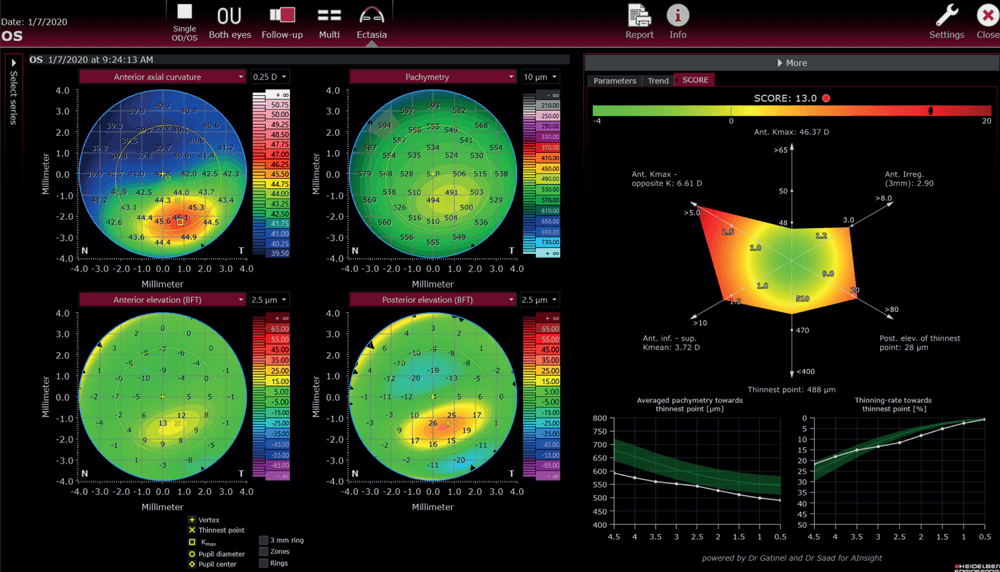
Around 10 years ago, Gatinel and Saad developed a scoring system for detecting corneas susceptible to ectasia, the SCORE Analyzer (5, 6, 7, 8, 9). Based on this system, a similar SCORE Development system was developed for the ANTERION. Gatinel and Saad analyzed all variables available from the ANTERION and added some additional criteria (such as inferior/superior keratometry, steepest/opposite keratometry, averaged pachymetry, and pachymetry thinning rate). Those metrics were then combined in a linear discriminant function, and multiplied by a coefficient, to achieve the required score that would separate the forme fruste from the normal corneas. As a result, Gatinel and Saad have been able to detect 75 percent of suspicious corneas – although Gatinel notes that the remaining 25 percent of eyes might not end up being affected by keratoconus, especially when there is no eye rubbing involved.
Heidelberg Engineering’s ANTERION multimodal imaging platform optimized for the anterior segment, which uses high-resolution SS-OCT, is regularly used by experts in the anterior segment field, in clinics around the world. Independent evaluations conducted by surgeons in their practices confirm that comprehensive measurements provided by the platform are invaluable in transforming cataract and refractive surgery, and cornea diagnostics. As Gundersen comments, modern cataract and refractive surgery cannot exist without accurate biometry measurements, and ANTERION is the platform of choice for accuracy, which gives better solutions to surgeons, and – ultimately – benefits their patients.
References
- AD Fişuş et al., “Comparison of 2 swept-source optical coherence tomography-based biometry devices,” J Cataract Refract Surg, 47, 87 (2021). PMID: 32769752.
- AD Fişuş et al., “Repeatability of 2 swept-source OCT biometers and 1 optical low-coherence reflectometry biometer,” J Cataract Refract Surg, 47, 1302 (2021). PMID: 33770018.
- B Gjerdrum et al., “Refractive precision of ray tracing IOL calculations based on OCT data versus traditional IOL calculation formulas based in reflectometry in patients with a history of laser vision correction for myopia,” Clin Ophthalmol, 15, 845 (2021). PMID: 33664562.
- IC Ramos et al., “Variability of subjective classifications of corneal topography maps from LASIK candidates,” J Refract Surg, 29, 770 (2013). PMID: 23980708.
- A Saad, D Gatinel, “Topographic and tomographic properties of forme fruste keratoconus corneas,” Invest Ophtalmol Vis Sci, 51, 5546 (2010). PMID: 20554609.
- A Saad et al., “Retrospective testing of a new method for detecting ectasia-susceptible corneas,” J Cataract Refract Surg, 37, 1907 (2011). PMID: 21930050.
- D Gatinel, A Saad, “The challenges of the detection of subclinical keratoconus at its earliest stage,” Int J Keratoco Ectatic Corneal Dis 1, 36 (2012).
- A Saad, D Gatinel, “Association of corneal indices for the detection of ectasia-susceptible corneas,” J Refract Surg, 28, 166 (2012). PMID: 22373030.
- C Chan et al., “Validation of an objective scoring system for forme fruste keratoconus detection and post-LASIK ectasia risk assessment in Asian eyes,” Cornea, 34, 996 (2015). PMID: 26165793.
