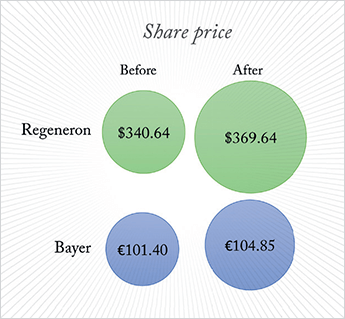
It’s interesting to see the results of an independently-run Phase III clinical trial first reported in a press release (1) by one of the companies whose drug was involved, ahead of peer-review publication – or even presentation at a congress. That’s exactly what’s happened in the case of the Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol T trial (2). Three drugs were evaluated: aflibercept, bevacizumab and ranibizumab, in patients with diabetic macular edema (DME) and visual acuity (VA) of between 20/32 and 20/320 (Figure 1). Regeneron, who market aflibercept in the US, “felt they had a fiduciary duty to release limited statements regarding the outcomes prior to the publication of the entire peer-reviewed data” (3). According to Regeneron’s release, aflibercept use resulted in a significantly greater improvement in mean change in best-corrected visual acuity (BCVA) from baseline at 52 weeks (the study’s primary endpoint) compared with both bevacizumab and ranibizumab intravitreal injections. The share prices of both Regeneron, and Bayer HealthCare (who have exclusive ex-US rights to aflibercept) rose on the news (Figure 2).
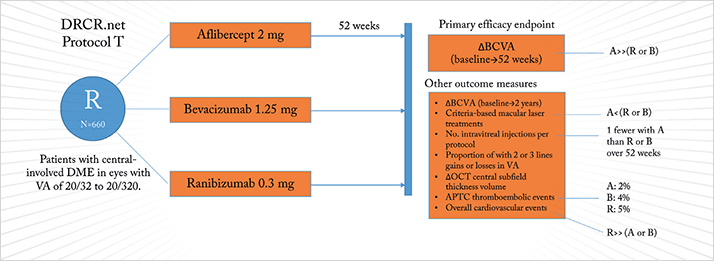
In terms of safety, ocular and systemic adverse event rates were similar across the three treatment arms of the study, although the rates of Anti-Platelet Trialists’ Collaboration (APTC)-defined arterial thromboembolic events (non-fatal stroke, non-fatal myocardial infarction, and vascular death) in the trial were 2, 4 and 5 percent in the aflibercept, bevacizumab and ranibizumab treatment arms, respectively. According to the release, there were also significantly more overall cardiovascular events in the ranibizumab arm than the bevacizumab or aflibercept arms (p<0.01), including a greater number of both cardiac and cerebrovascular events in the ranibizumab group. It would appear that DRCR.net is “currently in the process of finalizing and verifying the data prior to submission for publication” and they “intend to present the final results at a future medical conference simultaneous to, or soon after, publication” (1). Of note, “the DRCR.net will not be publicly discussing the results prior to publication” – which is why it’s surprising that Regeneron are (1).
Novartis, ranibizumab’s ex-US distributor “is aware that the topline results of the Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol T”, and point to ranibizumab’s “well-characterized safety profile, with more than 2.8 million patient-treatment years of exposure globally” (4). The US distributor, Genentech, put out a stronger statement: “We believe that the findings of a single trial at one year must be viewed in context of the totality of evidence establishing the efficacy and safety profile of Lucentis. During the first year of the Protocol T trial, patients in all study arms received a similar number of injections and experienced no significant difference in the percentage of serious adverse events including hospitalizations, death, or endpoints as represented by the APTC (non-fatal stroke, non-fatal myocardial infarction, and vascular death) criteria” (5). They added that “these preliminary data include a new and not yet validated endpoint of ‘Any Cardiovascular Event’ which contains a broad range of symptoms with unclear relationship to anti-VEGF drug use. This endpoint indicates a potential difference in cardiovascular events among the three medicines. Whether this is a real or chance finding is uncertain,” and “Caution is warranted with interpretation of the preliminary efficacy and safety results ahead of a full presentation and final peer-reviewed publication by the DRCR.net.”
References
- Regeneron Pharmaceuticals Inc. Press release, “EYLEA® (aflibercept) Injection Demonstrates Significantly Greater Gains in Visual Acuity than Both Bevacizumab and Ranibizumab in NIH-Sponsored Diabetic Macular Edema Study”, bit.ly/eyleadme. Accessed November 3rd, 2014. J.A. Wells for DRCR.net, “The Diabetic Retinopathy Clinical Research Network. Protocol T. Comparative Effectiveness Study of Aflibercept, Bevacizumab and Ranibizumab for DME”, bit.ly/protocolT. Accessed November 3rd, 2014. American Society of Retina Specialists, “Top-Line Data From DRCR.net Protocol T: Regeneron Statement”, bit.ly/asrsregeneron. Accessed November 3rd, 2014. Novartis AG. Press release, October 16, 2014, “Lucentis Efficacy and Safety in DME”, bit.ly/novartisdme. Accessed November 3rd, 2014. American Society of Retina Specialists “Top-Line Data From DRCR.net Protocol T: Genentech Statement”, bit.ly/protocolTgenentech. Accessed November 3rd, 2014.
