By definition, primary and emergency care physicians, although trained to deal with ocular injuries, are not ophthalmologists. So when patients present to them with eye injuries requiring urgent care, it can represent a serious challenge to these physicians, who lack the equipment and specialist training needed to accurately diagnose and assess the severity of the damage. Take, for example, postoperative glaucoma patients and patients with suspected penetrating eye injuries. Rupture of the anterior globe can occur in both cases, and is typically assessed with the Seidel Test. Even though it’s a simple test to perform, it’s a subjective test, and the amount of pressure and technique used varies across clinicians of all types. If you’re not both confident and experienced in using the test, this can lead to inaccurate assessments (1).
However, a team of US scientists, led by a bioengineer and an ophthalmologist, are working to create something better, and it comes in the form of a simple, point-of-care ascorbic acid sensor. As the aqueous humor contains around 20 times more ascorbic acid than the ocular tear film, high levels of ascorbic acid in the tear film act as a surrogate biomarker for the integrity of the anterior globe following eye trauma or surgery – and the more penetrating the injury, the higher the concentration of ascorbic acid can be detected in the aqueous humor.
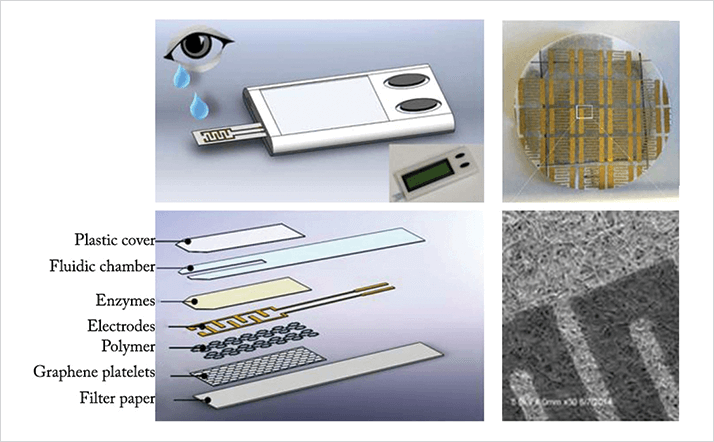
The sensor is comprised of a multilayer disposable test strip that contains ascorbate oxidase-coated gold electrodes, graphene, filter paper and an amphiphilic block copolymer (Figure 1). When the strip is applied to the tear film, the ascorbic acid present becomes oxidized by ascorbate oxidase, which induces a change in how the copolymer and graphene interact, causing a change in electrical resistance that can be detected. The device has so far demonstrated accuracy, sensitivity and specificity rates of over 80, 88 and 71 percent, respectively (2). Although the biosensor is currently at the prototype stage, the final product will be battery-operated, portable, should be easily operated by healthcare staff without specialized training and will display test results on an electronic screen. Its creators envision that the device will be of greatest utility in rural hospitals, at the site of accidents, or even on the battlefield, and will have applications in not only eye trauma cases, abut also suspected complications following trabeculectomy. “The new device will change the standard of care for evaluating eye traumas,” says Leanne Labriola, co-developer of the device and an ophthalmologist at Carle Foundation Hospital, Illinois, USA.
References
- WN May et al., “Standardized Seidel test to evaluate different sutureless cataract incision configurations”, J Cataract Refract Surg, 36, 1011–1017 (2010). PMID: 20494775. MR Fartia et al., “Point-of-service, quantitative analysis of ascorbic acid in aqueous humor for evaluating anterior globe integrity”, Sci Rep, 5, 16011 (2015). PMID: 26525715.
