- Transcorneal electrical stimulation (TES) appears to improve retinal function in patients with RP
- This may be due to TES increasing expression of neurotrophins and angiogenic signaling molecules
- Well-designed proof-of-concept clinical trials have demonstrated that TES is safe and can improve vision in patients with RP. Initial results from a larger study have confirmed the favorable safety profile of TES
- If TES manages to make it to the clinic, it represents something that’s increasingly rare in medicine: the ability to treat the (previously) untreatable.
Electrical stimulation of neural tissue is not exactly a new idea. Luigi Galvani was making frogs legs contract with electricity in 1792 (1), and in 1873, Henri Dor touted electrical stimulation as a remedy for all kinds of disorders – including eye diseases like retinitis pigmentosa (RP), glaucoma, amblyopia and optic atrophy (2). It didn’t last – therapeutic electrical stimulation was soon forgotten as more conventional fields of medicine advanced.
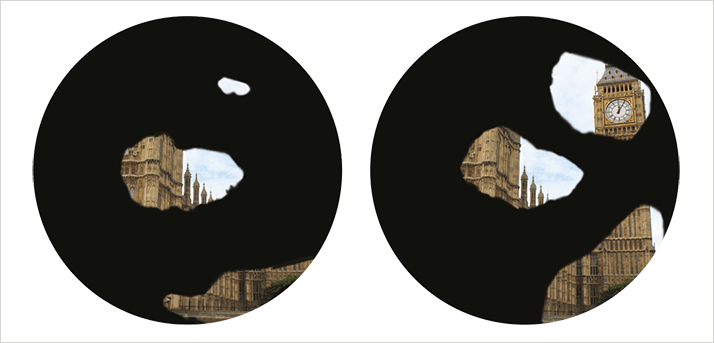
Transcorneal electrical stimulation (TES) became a focus of attention again in the late 20th century, but its use was limited to neurological research into the visual system, performed in animal models. Its potential therapeutic use was initiated in 2001 in patients who had received retinal implants. My team at the University of Tübingen’s Centre for Ophthalmology was performing research using a retinal prosthesis when we discovered that subthreshold currents – too small to evoke non-light- related visual perceptions known as phosphenes – improved visual function in patients with end-stage RP (see Figure 1) (3).
What was causing this improvement? It wasn’t the presence of a foreign body, something that was easily controlled for. Rather, multiple studies (4,5) demonstrated that the improved visual function induced by electrical stimulation was being driven by the expression in the eye of a mixture of neurotrophins (6). Roles in the process have been demonstrated for brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), insulin-like growth factor 1 (IGF-1) and fibroblast growth factor 2 (FGF-2) as well as for the anti-apoptotic protein, B-cell lymphoma 2 (Bcl-2). FGF-2 has also been implicated in angiogenesis; this is particularly interesting, as patients with RP have impaired choroidal function and morphology, and retinal electrical stimulation has been shown to increase choroidal blood flow (6, 7). An analysis of the RNAs expressed in the retinas of rats that received TES has shown that TES is associated with an upregulation of genes involved in embryonic, tissue and organ development pathways (7).
As RP is both incurable and confers a poor prognosis on diagnosis, our discovery generated significant interest in the ophthalmology community. A solid cohort of researchers have confirmed and extended our work, generating a clearer understanding of the mechanisms that underpin the therapeutic effect. To determine whether electrical stimulation can be of benefit to patients, studies were undertaken during 2007 and 2008 on diverse diseases including traumatic optic neuropathy, open angle glaucoma, age-related macular degeneration, and retinitis pigmentosa (8).
Proof-of-concept studies
Back in 2007–8 there had been no properly designed clinical trials of this technique. Although previous clinical evaluations of TES had shown improvements in visual function, they had been anecdotal in nature and not randomized, controlled studies. We initiated several pilot studies to investigate the effect of TES in patients with RP, primary open-angle glaucoma, Stargardt disease, or retinal arterial occlusion (RAO) (9).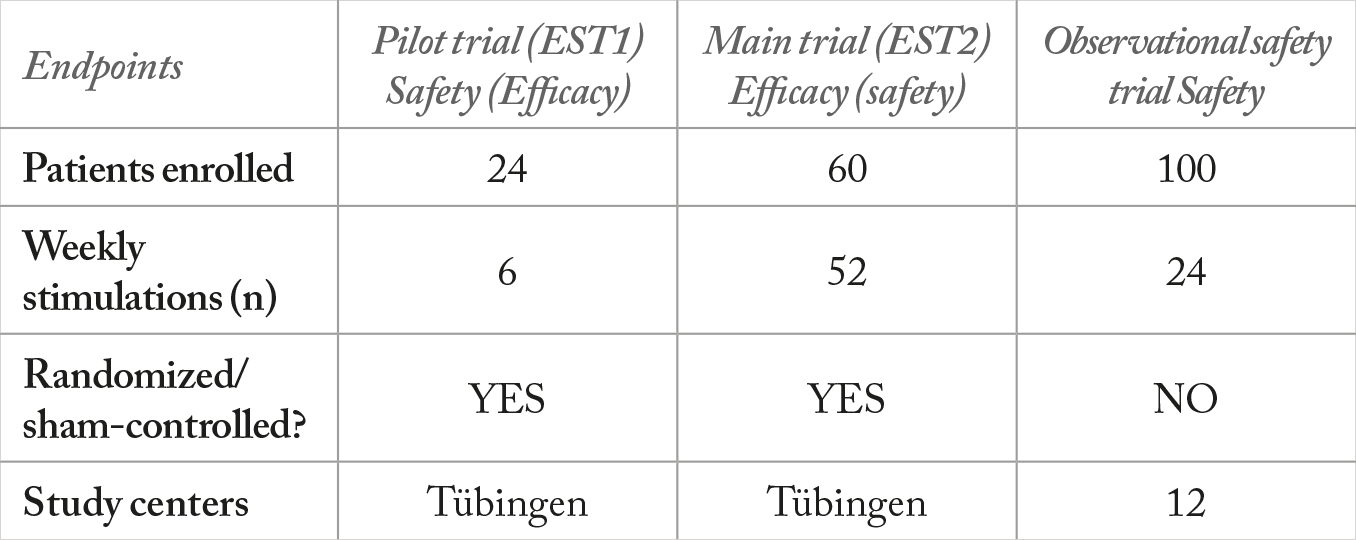
We performed two proof-of-concept trials: a multicenter open-label surveillance trial and an efficacy trial, named EST1 (see Table 1). The surveillance trial, which is still ongoing, involves 100 patients with RP. The efficacy trial involved randomization of patients to three groups: a sham group, and groups given 66 percent or 150 percent of the individual trial member’s electrical phosphene threshold (EPT). TES can induce phosphenes (Figure 2a) above a stimulation threshold that is specific to each individual. This threshold increases as RP progresses (10). We accounted for this variability by adjusting the stimulation intensity to each individual’s phosphene threshold, thus tailoring the treatment to each patient (6). TES was performed with an OkuVision GmbH OkuStim device for 30 minutes per week for six consecutive weeks (Figure 2b). Patients were checked for visual acuity, elaborate electroretinography, and visual field, among many other parameters (3).
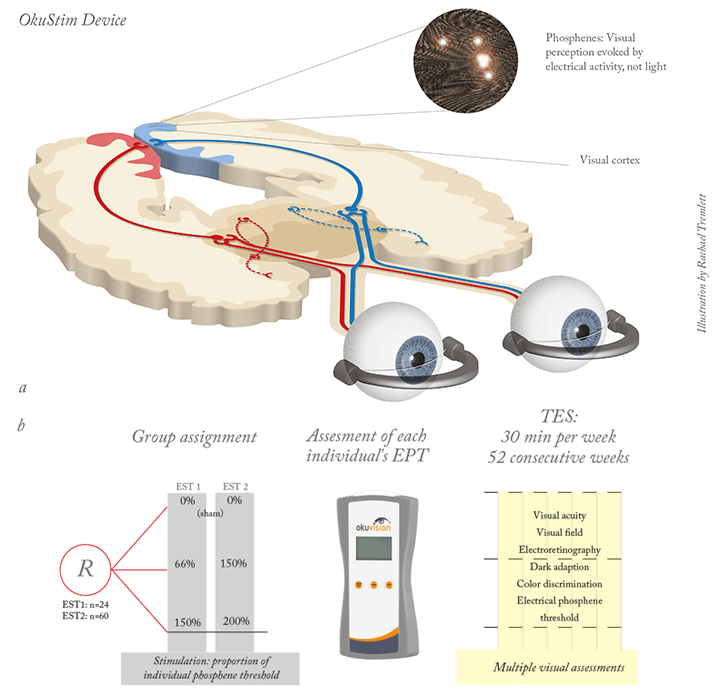
Significant beneficial effects were seen in patients in the 150 percent EPT group after six weeks. There was an increase in the dark-adapted ERG b-wave amplitude in this group compared with the sham control group, reflecting an improvement in the function of rods – the photoreceptor type that typically is most affected in RP. Additionally, RP almost always results in narrowing of the visual field and visual field area was significantly increased in the 150 percent threshold stimulation group compared with the sham controls. Given the results of the RNA transcript analyses performed in TES-treated rats, and the growth factors previously identified, we speculated that these improvements emanate from revitalized dormant retinal cells, the induction of new cell growth, or a reduced threshold of electrical activation in the remaining photoreceptors. More work is needed to assess these possibilities. The next step was to validate these results in a larger trial (EST2) with a greater number of patients treated over a longer time-period. In 2010 we began enrolling 60 patients with RP to be treated weekly with TES for a period of one year (Figure 2b). The last patient completed the study at the end of August this year and data evaluation is currently underway. We presented results of the safety analyses at EURETINA 2013, and our final results are due to be presented at ARVO 2014. EST2 demonstrated that the clinical application of TES has an acceptable safety profile. Adverse events were limited to minor eye pain and galvanic shock, and up to 30 percent of patients complained of dry eye, although this was controllable with artificial tears. Serious adverse events were all non-ophthalmological, and no other increases in any adverse events were observed in patients who received TES compared with the sham controls. No differences in visual acuity were observed between the treatment groups either. In conclusion, the OkuStim TES device has been used safely and effectively in patients with RP, and currently ongoing studies will better determine if electrical stimulation of the eye can reverse the retinal degeneration that occurs in patients with RP. The device should also be evaluated in patients with other ocular diseases that involve retinal damage, such as age-related macular degeneration, glaucoma and Stargardt disease. If the device successfully completes its clinical trials and makes it to the clinic, its impact could be substantial; it would offer hope to patients with currently untreatable disease.
Florian Gekeler is the Medical Director of the Eye Hospital of the Klinikum Stuttgart, Germany, and is a research consultant at the University of Tübingen.
References
- L. Galvani. “De viribus electricitatis in musculari motu”, cum Joannis Aldini dissertations et notis. Modena (1792). H. Dor. “Beiträge zur Electrotherapie der Augenkrankheiten”, Graefes Arch. Clin. Exp. Ophthalmol., 19, 316–352 (1873). M. T. Pardue, et al. “Neuroprotective effect of subretinal implants in the RCS rat”, Invest Ophthalmol Vis Sci. 46, 674–82 (2005). T. Fujikado, T. Morimoto, K. Matsushita, et al., “Effect of transcorneal electrical stimulation in patients with nonarteritic ischemic optic neuropathy or traumatic optic neuropathy”, Jpn. J. Ophthalmol., 50, 266–273 (2006). K. Inomata, K. Shinoda, H. Ohde, et al., “Transcorneal electrical stimulation of retina to treat longstanding retinal artery occlusion”, Graefes Arch. Clin. Exp. Ophthalmol., 245, 1773–1780 (2007). A. Schatz, T. Röck, L. Naycheva, et al. "Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study." Invest. Ophthalmol. Vis. Sci. 52 (7), 4485–96, (2011). G. Willmann, K. Schäferhoff, M. D. Fischer, et al., “Gene expression profiling of the retina after transcorneal electrical stimulation in wild-type Brown Norway rats”, Invest. Ophthalmol. Vis. Sci., 52, 7529-37 (2011). F. Gekeler, A .Messias, M. Ottinger et al., “Phosphenes electrically evoked with DTL electrodes: a study in patients with retinitis pigmentosa, glaucoma, and homonymous visual field loss and normal subjects”, Invest. Ophthalmol. Vis. Sci., 47, 4966–74 (2011). L. Naycheva, A. Schatz, T. Röck, et al., “Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retina diseases”, Invest. Ophthalmol. Vis. Sci., 53, 7440–8 (2012). F. Gekeler, A. Messias, M. Ottinger, et al., “Phosphenes electrically evoked with DTL electrodes: a study in patients with retinitis pigmentosa, glaucoma, and homonymous visual field loss and normal subjects”, Invest. Ophthalmol. Vis. Sci., 47, 4966–74 (2006).
