
Although around 200,000 corneal transplants are performed worldwide each year – and corneal transplant is the most common organ transplant around the globe – close to 13 million people are still on a waiting list for a corneal graft (1), with average delays measured in years.
EyeYon Medical, founded by two leading cornea specialists – Ofer Daphna and Arie Markovich – and medical device entrepreneur Nahum Ferera, decided to address the shortage by developing EndoArt®. The ready-to-use, universally-available endothelial graft essentially replaces endothelial keratoplasty (DSAEK and DMEK) as the first line of treatment for corneal edema. EndoArt® may eliminate the waiting list for donor corneas, as well as the risks associated with transplanting tissues.
The EndoArt® implant is dome shaped, around 50 microns thick, with a curvature that resembles the posterior part of the cornea. It is made of an optically clear, foldable, biocompatible co-polymer with the mechanical strength to allow smooth and controlled insertion and easy intraocular manipulation – without the risk of damaging the implant (a potential issue with donor tissue).
The end result: a strong, resilient graft that covers around 40 percent of the cornea’s area, blocking excessive aqueous humour penetration from one side, while ensuring corneal nutrition from the other. Moreover, continual fluid evaporation from the anterior part of the cornea helps to balance the cornea’s water content. As EndoArt® was designed to impede water permeability to compensate for the reduced efficacy of the endothelial pump, it is applicable to mitigating corneal edema regardless of etiology.
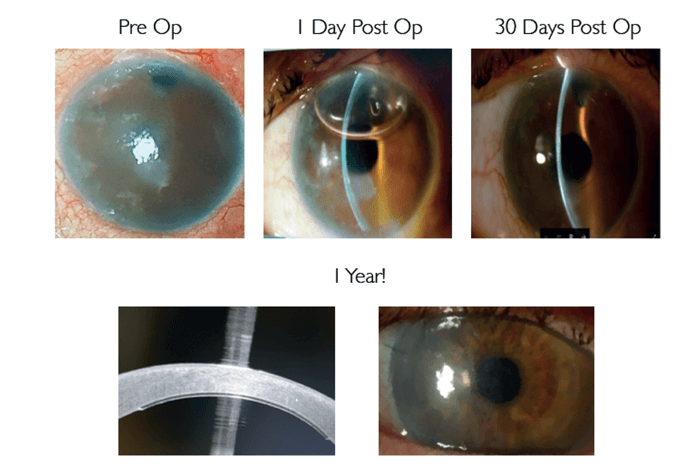
From a surgical point of view, the folded implant is inserted into the eye’s anterior chamber through a clear corneal incision, typically around 2.2 mm in length (such small incisions are usually self-sealing, requiring no sutures). After insertion, EndoArt® unfolds itself and is attached to the posterior corneal surface using the air bubble technique, similar to a DSAEK or DMEK procedure; at this stage, it is possible to manipulate the implant as needed, which results in an easier and safer surgical technique. Corneal surgeons who have performed endothelial keratoplasty (EK) will be able to implant EndoArt® without any special training. And cataract surgeons who are not trained in EK should only require few training sessions to master the technique.
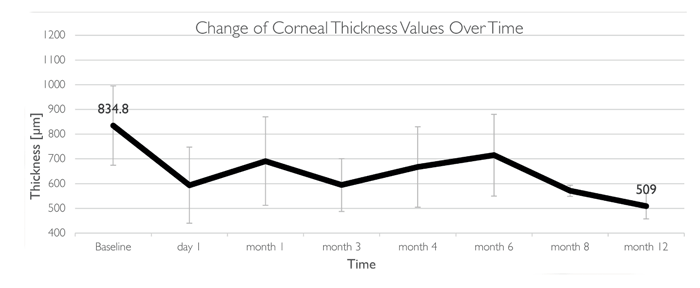
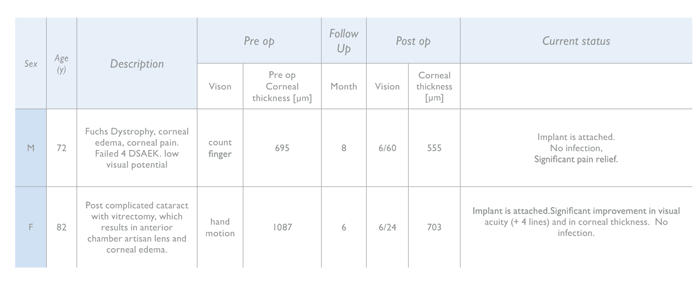
The implant is currently undergoing a first-in-human, multi-center trial across Europe, Asia and Israel, with 14 patients enrolled and a follow up of 15 months (2). With more than one year of follow-uptime completed, it has already demonstrated both safety and feasibility in humans. All patients demonstrated decrease in corneal thickness and in patients with visual potential, there has been an improvement in visual acuity, with no evidence of nutrition issues.
Ruth Lapid-Gortzak, trial PI, from the Amsterdam University Medical Center, reports that the EndoArt® is a paradigm shift in corneal transplantation. “This device opens up a new vision for the future: availability, ease of use and cost reduction in treating patients with endothelial failure. It is a very welcome addition to the arsenal of corneal surgery,” she says. “The initial results are very encouraging. The patients and the implanting team are very enthusiastic. Patients recover vision very swiftly after the procedure (in eyes that had some visual potential, as in the trial, for ethical reasons, we implant eyes with guarded visual prognosis). Technically, this is swifter and easier than DSEK, DSAEK and DMEK. At this time, we have three patients with more than 6 months follow up, of which two have recovered vision.”

Gerd Auffarth, Professor and Chairman of The Department of Ophthalmology, Ruprecht-Karls University of Heidelberg, says: “For decades researchers were looking for an artificial whole graft cornea. Since 60 to 70 percent of today’s corneal transplant surgery is DMEK, we actually need an artificial DMEK lamella. I now have more than one year’s experience with the EndoArt® Artificial DMEK lamella. To date, two patients have received the implant and both have performed very well so far. The implant works by blocking inflow of aqueous humor into the cornea, thus reducing corneal edema.”

The EndoArt® is the first and only off-the-shelf artificial product for partial keratoplasty and, as such, it represents a new avenue to treat the root cause of corneal edema.
References
- P Gain et al., “Global survey of corneal transplantation and eye banking,” JAMA Ophthalmol, 134, 167 (2016). PMID: 26633035.
- Clinical Trials, “Study to evaluate the safety of the EndoArt for treatment of subjects suffering from corneal edema.” Available at: https://bit.ly/3i6WkZA.
