Early signs of corneal asymmetries are often visible on the posterior surface first – and that’s why the reliable measurement of both anterior and posterior surfaces
is vitally important. GALILEI, a diagnostic device from Ziemer, is able to make all the measurements required for cataract and refractive surgery planning in a single session, making it a truly complete solution for ophthalmic surgeons.
Placido topography, Dual-Scheimpflug tomography, and optical biometry, with all measurements aligned to first Purkinje reflex, are combined within the GALILEI device to
assist surgeons in reliably measuring the anterior and posterior surfaces, as well as the eye’s axial length. The system’s two Scheimpflug cameras provide precise pachymetry and elevation data, which aid in the early detection of asymmetry and bulging.

Renowned cornea and refractive surgeon Shady Awwad, Associate Professor of Clinical Ophthalmology and Director of the Refractive Surgery Center at the American University of Beirut, uses GALILEI’s Dual-Scheimpflug to screen candidates for laser vision correction.
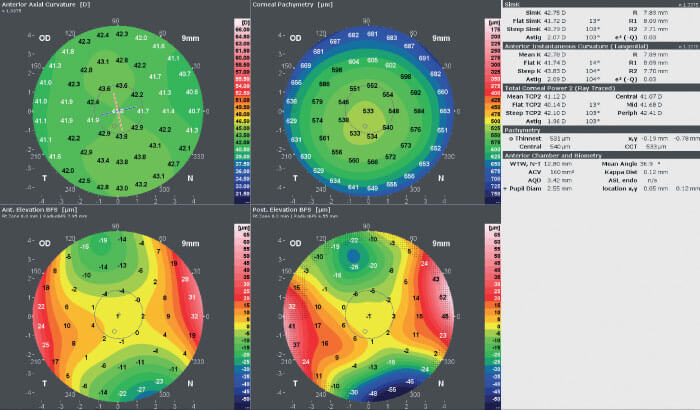
For the last few months he has been using GALILEI’s latest report, to be added shortly: the Corneal Thickness Progression Report. The report, which consists of three x/y graphs, four maps, and a set of indices, compares the patient’s measured data with data from the normal population, and shows the change in corneal thickness towards the periphery relative to the thinnest point. Awwad says, “I believe the Corneal Thickness Progression Report is one of the most sensitive tools to help detect early ectasia. I start the analysis by looking at the captured image, to make sure the measurement is unobstructed and centered, and to assess the position of the measurement center vis-à-vis the center of the cornea.” Awwad then evaluates the “Quadmap” (the anterior and posterior instantaneous curvature, posterior Best Fit Sphere, and pachymetry maps) (see Figure 2) before checking the Corneal Thickness Progression Report.
Awwad says the report is invaluable when planning his surgeries: “The Corneal Thickness Progression Report is key in identifying very early ectatic corneas (keratoconus suspects), and I have found it to be very sensitive and capable of producing specific data. It often shows values positive for early ectasia when other traditional parameters show normal to borderline levels. I believe it provides an extra safety net in making the decision whether or not to proceed with laser vision correction.” (see Figure 3).
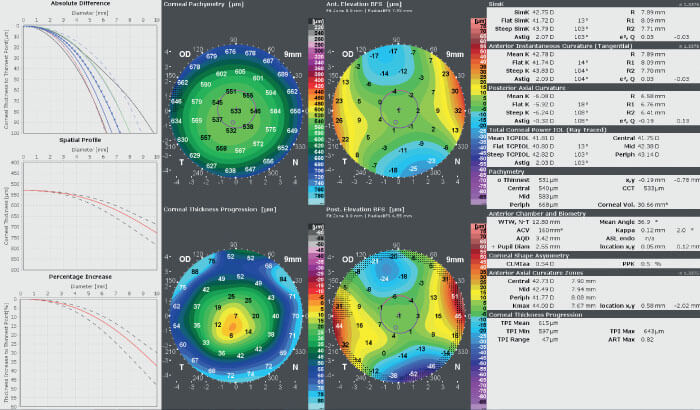
In addition to the x/y graphs, the Report includes a Corneal Thickness Progression map, which visualizes the data in an easy-to-read map. It is unique, as it provides surgeons with the precise location of suspicious regions within the cornea. Awwad says, “The Corneal Thickness Progression map displays the rate of change in corneal pachymetry with respect to the thinnest point at any given point in the cornea. Whereas the x/y graphs average corneal thickness change at a given distance from the thinnest point and over all meridians. The problem with the latter approach is that early abnormal pachymetric changes, typically focal, will be diluted by the system, averaging them with other areas in the cornea. Instead, the Corneal Thickness Progression map can help the clinician depict an early focal pachymetric change, typically infero-temporal or inferior, that is faster than in the rest of the corneal areas, and potentially faster than the expected normal range. This has been shown to be one of the earliest manifestations of ectasia.”
In summary, the additional information provided by the GALILEI helps clinicians with diagnosis and decision making – thus offering solid support in refractive surgery planning. Shady Awwad draws a strong conclusion: “The Corneal Thickness Progression Report is a boon for ophthalmologists involved in laser vision correction. Used in combination with other parameters, it allows us to make confident surgical decisions.”
The GALILEI G4 is CE marked and FDA cleared. The GALILEI G6 is CEmarked and pending FDA clearance. For some countries, availability may be restricted due to regulatory requirements. Please contact Ziemer for details.
