A Brief History of IOL Materials
The development and use of intraocular lenses has been a major ophthalmic success story. Here, we describe some of the key contributions and the materials that revolutionized cataract treatment.
At a Glance
- Cataract surgery stretches back three thousand years
- Harold Ridley introduced the modern era in 1949, with an intraocular lens made from poly(methyl methacrylate)
- Foldable IOLs, requiring much smaller incisions, came to dominate, especially silicone IOLs in the 1980s
- Patients in the developing world are often denied these superior IOLs on grounds of cost
Cataract surgery, the most commonly performed surgical technique in the world today, has an illustrious history. The earliest example, cataract “couching”, was first reported around three thousand years ago in India in their ancient text, the Mahabharata. Couching is a procedure that should be consigned to historical textbooks: the pressing down of cloudy lenses into the vitreous with a thorn or a needle. It leaves the patient aphakic (but with some visual function) requiring a powerfully positive prescription lens to compensate. While unthinkable in most part of the world, it still occurs today in some rural regions of Sub-Saharan Africa, and is performed by local healers rather than by ophthalmologists.
Progress in recent times has been rapid, such that modern surgical techniques for extracting the human lens appear to be nothing short of astonishing when compared to what was performed just thirty years ago. Incision size reductions and the use of phacoemulsification have revolutionized cataract surgery. This has been a second revolution; the first was the invention of an implantable, well tolerated and technically feasible lens. Here we tell the story of the intraocular lens (IOL), from the shattered canopy of a Second World War aircraft to where we are today.
Origins
The true beginning of the IOL is the Second World War. Against a backdrop of the Battle of Britain, where aircraft fought for air supremacy in the skies over the south of England, Harold Ridley was a civilian ophthalmologist who operated on Royal Air Force pilots with eye injuries. On August 15, 1940, inspiration stuck. A pilot’s Perspex canopy had shattered, sending numerous splinters of poly(methyl methacrylate) (PMMA, Figure 1) into his eyes. Ridley performed a total of 19 operations on the pilot, saving the vision in one eye. During the process, he realized that the body’s immune system had not reacted against the PMMA splinters; unlike glass splinters, they remained inert in the eye. Ridley recognized that this material could be used for artificial lenses, and that these could be implanted into the eye to replace the natural lenses removed during cataract surgery (1-3).
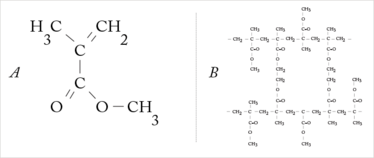
Figure 1. (a) MMA (methyl methacrylate) forms the basis for acrylic IOLs. (b) Poly(methyl methacrylate) (PMMA) is a transparent thermoplastic; it was initially developed as a lightweight and shatter-resistant alternative to glass.
He worked with the Rayner Optical Company and together they used ICI’s Transpex I, a high quality version of PMMA, to produce the first IOL (Figure 2). On November 29, 1949, at St Thomas’ Hospital, London, Ridley inserted the first lens into a 42-year-old woman after an extracapsular cataract excision (2–6).
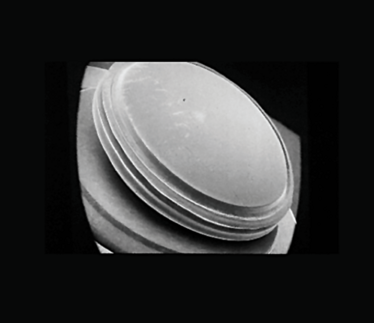
Figure 2. Scanning electron micrograph of a Ridley intraocular lens made from PMMA.
While history views this as a pivotal moment in the development of the field, many in the ophthalmic establishment at the time strongly disapproved of Ridley’s work. Nevertheless, others followed his example, including Warren Reese, the first American to implant an IOL, and the Ridley-designed IOLs began to be implanted widely with great success. Complications did occur, including severe hyphema, downward decentration, iris atrophy, glaucoma, anterior and posterior dislocation, and inflammation, meaning that approximately 15 percent of the Ridley implants were eventually removed.
IOL design evolved and with it establishment disapproval waned, although it took until the 1970s before IOL implantation after cataract surgery was considered to be a standard procedure.
PMMA as the material used to make IOLs had many advantages (see "The pros and cons of PMMA" ), but it also had one major disadvantage: the corneal incision size required to implant it. As PMMA is rigid, the incision needed to be at least as big as the IOL, resulting in a large wound that needs to be stitched closed. This can induce astigmatism and, compared with modern cataract surgery, requires a prolonged recovery time. Another driver for smaller incision holes occurred when Charles Kelman introduced phacoemulsification in 1967; surgeons made a small incision for the phaco tip, but then had to enlarge it to place the lens. Something had to be done about the incision size – and that meant flexible, and therefore foldable, IOLs (1,7,8).
The pros and cons of PMMA
Pros
- Extensive clinical experience
- Suitable for single piece IOLs, three-piece lenses and IOL haptics
- Excellent biocompatibility
- Hydrophobic surface
- Outstanding optical properties – high light transmissibility
- Can add UV-absorbing materials
- Inexpensive
Cons
- Rigid – meaning that the incision size needs to be at least as big as the diameter of the IOL.
- The incision needs to be sewed shut – this can induce post-operative astigmatism
Foldable IOLs
The first foldable IOLs were developed in the 1950s, and were made of hydrogels. Hydrogels are hydrophilic networks of polymers that swell extensively on contact with water; they vary in size and properties, depending in part on their water content. In the hydrated state, hydrogels are flexible, clear, non-immunogenic and resemble living tissue – making them an excellent material to make foldable IOLs from (albeit a material that was considerably more expensive than PMMA). As water saturation determines hydrogel size, it means that you can implant a semi-hydrated lens through a small incision, and it will expand in the eye as it becomes fully hydrated.
Nevertheless, early foldable IOLs had issues with decentration (because they elbowed and folded as the capsular bag contracted), but this issue was practically eliminated by the use of continuous curvilinear capsulorhexis.
The first foldable silicone IOL (Figure 3) was implanted in human eyes in the 1978 by Kai-yi Zhou (9). These were rapidly adopted, and foldable silicone IOLs conquered the market in the 1980s. In 1989, AMO introduced the PhacoFlex model SI-18, the first commercially available three-piece silicone IOL platform for use after clear corneal small incision phacoemulsification. This was followed in 1997 by the first FDA-approved multifocal IOL, the Array (also manufactured by AMO), which also contained a silicone lens, and for a long time dominated the multifocal IOL market (1).
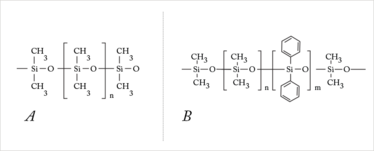
Figure 3. (a) Polydimethylsiloxane and (b) polydimethyldiphenylsiloxane. Silicone is a synthetic polymer constructed as an organic polysiloxane molecule. These molecules consist of periodically repeated silicon-oxygen-groups. This structure is the backbone for a polymer, which is identical for all silicone IOLs. Bound to the silicon atom are side chains, which influence the properties of the material. First-generation silicone materials (like polydimethylsiloxane) had methyl side chains. Second-generation silicones have the methyl side chains replaced with vinyl groups.
Besides smaller incision sizes, foldable IOLs were insertable using single-use applicators or implantation devices, making the procedure easier for the surgeon, and reducing the risk of ocular infection. An added bonus is that the incision sizes used when introducing foldable IOLs are so small relative to rigid lenses, they are normally self-sealing, produce less astigmatism, and allow faster visual rehabilitation.
Acrylic lenses can be flexible too. PMMA may be rigid, but the substitution of side-chain molecules to ones like hydroxyethyl or polyethyl groups (Figure 4) can generate flexible, clear acrylic materials for foldable IOLs. Furthermore, depending on the side-chain chemistry, the flexible acrylic material can be made to be hydrophilic or hydrophobic. Most hydrophilic IOLs utilize the same material as contact lenses: hydroxyethylethacrylate (HEMA), and with a water content of approximately 38 percent, it’s fairly flexible too.
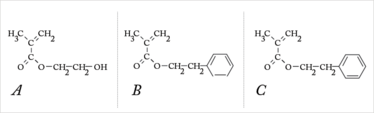
Figure 4. Flexible acrylic lenses can be made from (a) HEMA – (Hydroxyethyl) methacrylate, (b) PEMA – (polyethyl) methacrylate, and (c) PEA – Poly(ethyl acrylate).
A critical property of an acrylic material is glass transition temperature (Tg) – the temperature at which a material changes from a hard and brittle state to a more flexible state – and this varies by polymer structure. Accordingly, it’s important to bear in mind Tg when folding IOLs – if an IOL material has a high Tg, it’s important not to fold it in a cold environment.
Foldable acrylic lenses tend to be more robust than their silicone equivalents and to undergo less post-implantation decentration or rotation. If posterior segment surgery is likely to be necessary at a later date, they are a better choice, as silicone oil – which would ruin silicone-based IOLs – can be used. However, this comes at the cost of a slightly larger incision size being necessary for implantation.
Conclusion
Today, Ridley’s genius has improved the lives of many millions of people. IOL use is not limited to cataract surgery, they have become an important method of improving refractive outcomes, as part of clear lens exchange. The gradual improvement in IOL design, first in making flexible lenses, then the ever-improving optical outcomes have meant that vision after cataract surgery has never been better – in the developed world. Alas, in developing countries, if patients do receive an IOL, for cost reasons it is likely to be a rigid PMMA lens. We have come a long way in terms of IOL design, but many people with cataracts in rural areas of the developing world, need help to catch up.
Sibylle Scholtz is a Specialist on Ophthalmic History at the International Vision Correction Research Centre (IVCRC), Florian Kretz is an Ophthalmologist and Senior Research Specialist at the IVCRC and Gerd Auffarth is the Director of the David J. Apple International Laboratory of Ocular Pathology and IVCRC as well as Chairman of the Department of Ophthalmology, Ruprecht-Karls-University of Heidelberg, Germany.
- D.J. Apple, G.U. Auffarth, Q. Peng, N. Visessook, “Foldable Intraocular Lenses: Evolution, Clinicopathologic Correlations, and Complications”, Slack (Publ.), ISBN 1556424353, 9781556424359 (2000).
- G.U. Auffarth, D.J. Apple, “History of the development of intraocular lenses”, Ophthalmologe, 98, 1017–28 (2001).
- G.U. Auffarth, J. Schmidbauer, “Intraocular lenses”, Ophthalmologe, 98(11):1011 (2001).
- N.H.L Ridley, “Further experiences of intraocular acrylic lens surgery”, Br. J. Ophthalmol., 38, 156–162 (1954).
- N.H.L. Ridley, “Intraocular acrylic lenses – past, presence and future”, Trans. Ophthalmol. Soc. UK 84, 5–14 (1964).
- N.H.L. Ridley, “The cure of aphakia, 1949. Intraocular lens implantation”, Rosen, Haining and Arnott, C.V. Mosby, Toronto, 37–41 (1984).
- A.M. Beasley, G.U. Auffarth, A.F. von Recum, “Intraocular lens implants: a biocompatibility review”, J. Invest. Surg. 9, 399–413 (1996).
- G.U. Auffarth, M. Wilcox, J.C. Sims, et al. “Analysis of 100 explanted one-piece and three-piece silicone intraocular lenses”, Ophthalmology, 102, 1144–50 (1995).
- K.-Y. Zhou, “Silicon Intraocular Lenses in 50 Cataract Cases“, Chinese Medical Journal, 96, 175–176 (1983).
- D.J. Apple, “Sir Harold Ridley and his fight for sight: he changed the world so that we may better see it”, Slack (Publ.). ISBN 1-55642-786-7 (2006).

One of The Ophthalmologist’s Top 40 under 40 cadre, Florian is a lead surgeon at the Eyeclinic Ahaus-Raesfeld-Rheine, Ahaus Germany, as well as a consultant ophthalmologist and research fellow at the International Vision Correction Research Centre Network and David J. Apple International Laboratory for Ocular Pathology at the Department of Ophthalmology, University Hospital Heidelberg. When not in the clinic, lab, office, or on the autobahn, Florian enjoys spending time with his wife and young family.
Sibylle Scholtz is a Specialist on Ophthalmic History at the International Vision Correction Research Centre (IVCRC)
Professor and Chairman of the Department of Ophthalmology, University of Heidelberg; and Director of the International Vision Correction Research Centre, David J. Apple International Laboratory for Ocular Pathology, Germany.













