Even with the finest skills, technique, devices and experience, performance suffers when clarity is compromised

Nd:YAG laser capsulotomy is still the standard of care for the treatment of posterior capsule opacification (PCO). Up to 20 percent of pseudophakic patients undergo the procedure within five years after cataract surgery. Although the technique is minimally invasive, fast, and generally low-risk, potential side-effects do exist and are receiving increasing attention. Transient intraocular pressure (IOP) spikes, macular edema, inflammatory responses, and lasting optical degradation of the IOL have all been reported, particularly when the technique is improperly applied (1,2). Therefore, meticulous planning and execution of laser treatments are crucial. This includes precise laser focusing with a posterior offset, selecting the minimum effective energy, and avoiding the central 3-mm optic zone during treatment.
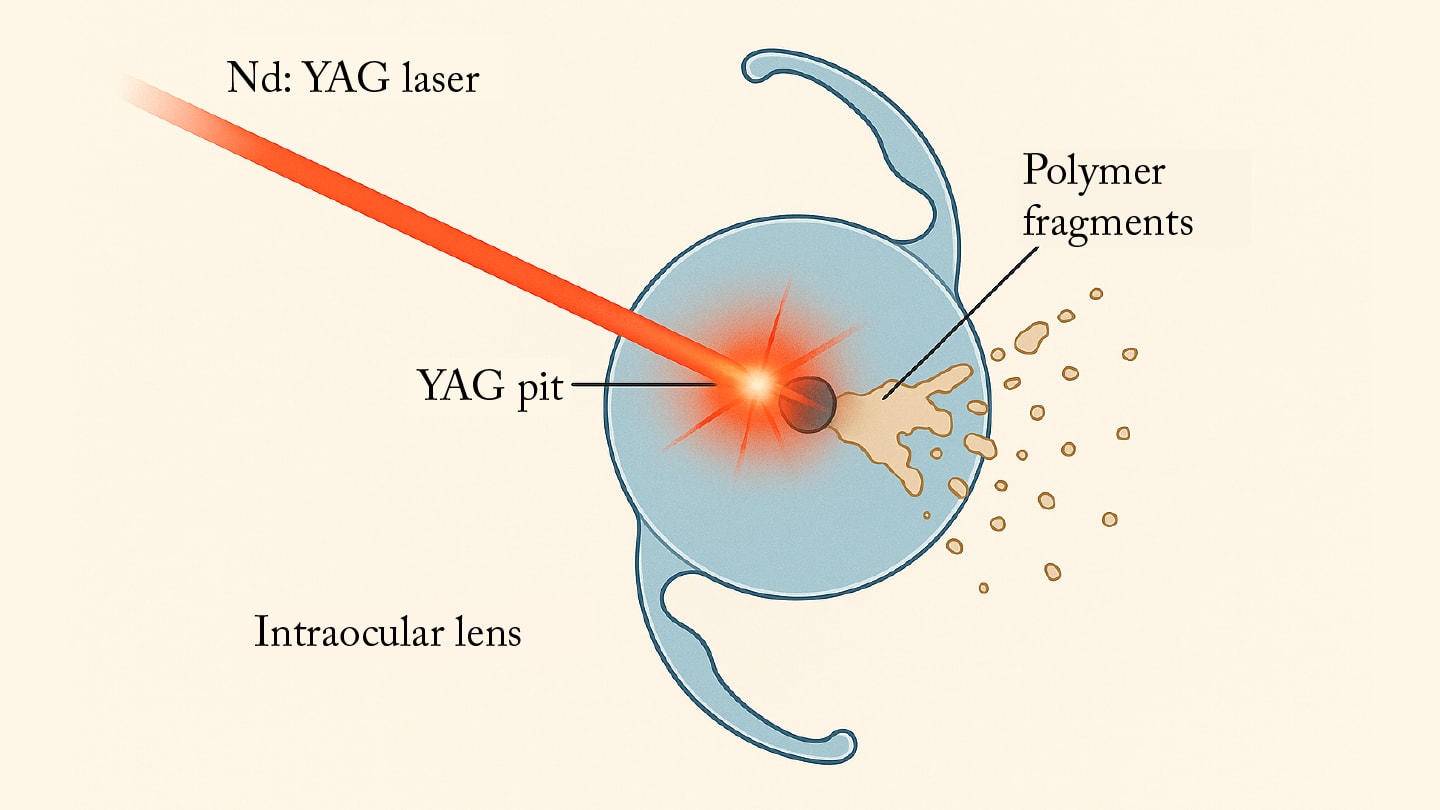
A commonly observed consequence of laser application is the formation of so-called “YAG pits” or “YAG shots” – microscopic craters resulting from misaligned or improperly focused laser pulses on the intraocular lens (IOL) optic (3). Numerous studies have shown that even a few centrally located YAG pits can significantly compromise the optical quality of an IOL (4-6). For several years now, our team has been investigating this issue in experimental studies, demonstrating that these defects can lead to a marked reduction in image contrast and a measurable increase in light scattering (7,8).
In multifocal IOLs, contrast losses of up to 48 percent within the visible spectrum have been observed (9). Further investigations revealed that centrally located defects, in particular, contribute to increased subjective glare, underscoring the clinical relevance of such damages. Our bench tests and simulations also confirmed that YAG-induced surface damage degrades the modulation transfer function (MTF) and impairs retinal image quality (4,8,9). This is especially relevant for patients with so-called premium IOLs (e.g., trifocal or small-aperture designs), where even minor optical imperfections can be visually significant.

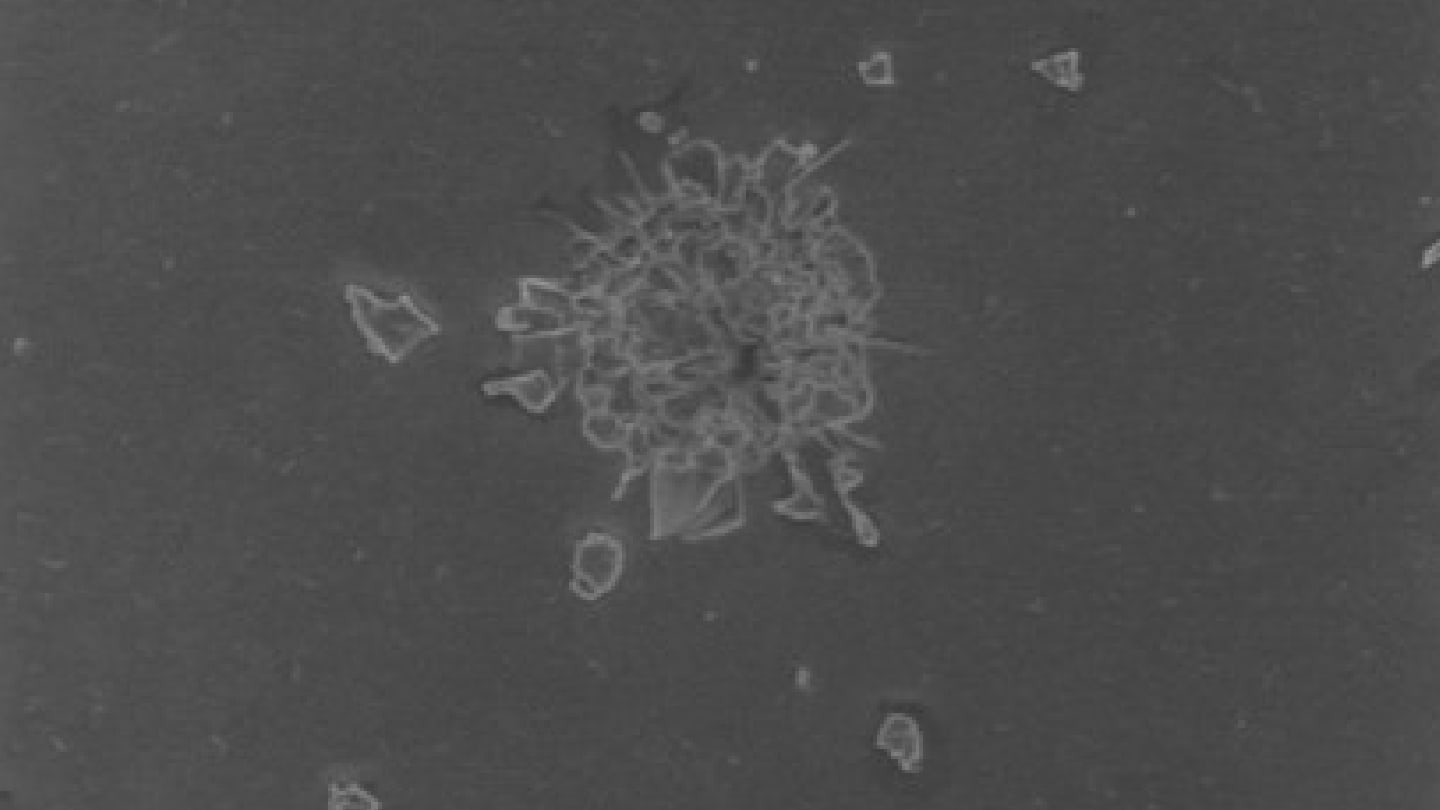
Against this already studied background, the question arises whether, in addition to structural damage, microscopically small polymer fragments may also be generated and dislodged from the lens material by the laser energy (Figures 2 and 3). It is hypothesized that such fragments could act as sources of stray light, inflammatory triggers, or contributors to pressure spikes. The aim of the recent study was to experimentally assess whether, under clinically relevant conditions, polymer fragments can be detached and mobilized from modern hydrophobic and hydrophilic acrylic IOLs (10).
In this study, six commercially available monofocal hydrophobic and hydrophilic acrylic IOLs were all “treated” under standardized conditions using single-pulse application (2.6 mJ) from an Nd:YAG laser (Visulas YAG). In all the IOL types we examined, microscopic fragments on the surface of the IOL – ranging from 10 to 20 µm – were detected following laser exposure. Raman spectra showed clear correspondence with the respective IOL polymers (10). After ultrasonic treatment, the particles were no longer visible on the lens surfaces but could still be found on the filters and were spectroscopically identified (Figure 4). Fragment release occurred irrespective of IOL material type. However, differences were observed in pit morphology: hydrophobic lenses tended to show deeper and more irregular craters with broader fragmentation zones.

These findings demonstrate that even with clinically standard energy settings, solid polymer fragments can be dislodged from IOLs. Although this was an in vitro study, potential in vivo implications are conceivable: free particles may contribute to elevated intraocular pressure, affect the angle structure, or trigger inflammatory reactions. Additionally, optical impairment due to increased light scattering remains a concern. Previous studies have reported macular edema (Irvine Gass syndrome) and transient IOP elevations of patients after cataract surgery and YAG capsulotomy in some cases (11). Whether – and to what extent – these polymer particles may also contribute remains unclear, but warrants further investigation.
Several parameters influence defect and fragment formation, including focal depth, pulse energy, pulse count, and beam convergence. Improper focusing – for instance, due to chromatic aberration between aiming beam and laser pulse – can shift the effective focal point by over 100 µm. Central YAG pits in multifocal or small-aperture IOLs are especially likely to result in clinically relevant visual disturbances.
This study is the first to show that microscopic polymer fragments can be generated and mobilized from IOLs under clinically relevant conditions. Spectroscopic analysis confirmed their chemical match with the IOL substrate. These findings emphasize the need for utmost care in laser focusing, energy selection, and pulse placement – in all cases, but particularly in premium IOLs (12). Future clinical studies should assess whether and how these particles affect visual quality, intraocular pressure, or inflammatory responses. The combination of microscopy, Raman spectroscopy, and filtration offers a new methodological framework for evaluating IOL damage in a translational context. Given these findings, utmost caution must be exercised when performing laser capsulotomy to minimize unintended IOL damage and its potential consequences.
References
- AF Borkenstein, EM Borkenstein, “Neodymium-doped yttrium aluminum garnet (Nd: YAG) laser treatment in ophthalmology: a review of the most common procedures Capsulotomy and Iridotomy,” Lasers Med Sci., 39, 167 (2024). PMID: 38954050.
- R Ling et al., “Evaluation of Nd:YAG Laser Capsulotomy Rates in a Real-Life Population,” Clin Ophthalmol., 14, 3249 (2020). PMID: 33116375.
- AF Borkenstein et al., “Incorrectly Focused Neodymium:Yttrium-Aluminum-Garnet (Nd:YAG) Laser Beam Leads to Massive Destructive Effects in Small-Aperture (Pinhole) Intraocular Lenses,” Ophthalmol Ther., 13, 2745 (2024).PMID: 39153117.
- AF Borkenstein et al., “Image Contrast and Spectral Transmission in Intraocular Lenses with Nd:YAG Pits,” Curr Eye Res., 48, 911 (2023). PMID: 37382106.
- AF Borkenstein et al., “A Novel 3D High Resolution Imaging Method Using Correlative X-Ray and Electron Microscopy to Study Neodymium-Doped Yttrium Aluminum Garnet Laser-Induced Defects in Intraocular Lenses,” Ophthalmic Res., 67, 292 (2024). PMID: 38718759.
- AF Borkenstein et al., “Micro-Computed Tomography (CT) as a Tool for High-Resolution 3D Imaging and Analysis of Intraocular Lenses: Feasibility and Proof of the Methodology to Evaluate YAG Pits,” Ophthalmol Ther., 12, 447 (2023). PMID: 36481844.
- AF Borkenstein, EM Borkenstein, “Analysis of YAG Laser-Induced Damage in Intraocular Lenses: Characterization of Optical and Surface Properties of YAG Shots,” Ophthalmic Res., 64, 417 (2021). PMID: 33221803.
- AF Borkenstein et al., “Evaluating impact of Nd: YAG laser associated defects on optical quality of hydrophilic and hydrophobic intraocular lenses using visualization of light propagation and USAF test targets,” BMC Ophthalmol., 22, 494 (2022). PMID: 36527032.
- AF Borkenstein et al., “Image Contrast and Spectral Transmission of Presbyopia-Correcting Intraocular Lenses: Evaluating the Impact of Nd:YAG Laser Associated Defects,” Curr Eye Res., 50, 51 (2025). PMID: 39139124.
- AF Borkenstein et al. “Nd:YAG Laser Induced Microfragmentation In Intraocular Lenses: A Correlative Optical and Raman Spectroscopy Study”, Curr Eye Res., submitted, under review (June 2025).
- LF Desideri et al. “Incidence, Pathogenesis, Risk Factors, and Treatment of Cystoid Macula Oedema Following Cataract Surgery: A Systematic Review,” Diagnostics, 15, 667 (2025). PMID: 40150009.
- AF Borkenstein, EM Borkenstein, “Precise Posterior Nd:YAG Capsulotomy without Creating Defects is Key for Patients' Quality of Vision,” Clin Ophthalmol., 18, 361 (2024). PMID: 38343902.
