Oxervate® (cenegermin-bkbj) is a topical formulation of recombinant human nerve growth factor combining elevated science with a grounded approach to the needs of neurotrophic keratitis (NK) patients.
This comprehensive new report, featuring case studies, ophthalmologist interviews, clinical trial data, and background information shows Oxervate® as a non-surgical intervention with unprecedented real-world impact, lauded both by ophthalmologists and those they care for.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Use with Contact Lens
Contact lenses should be removed before applying OXERVATE because the presence of a contact lens (either therapeutic or corrective) could theoretically limit the distribution of cenegermin-bkbj onto the area of the corneal lesion. Lenses may be reinserted 15 minutes after administration.
Eye Discomfort
OXERVATE may cause mild to moderate eye discomfort such as eye pain during treatment. The patient should be advised to contact their doctor if a more serious eye reaction occurs.
ADVERSE REACTIONS
In clinical trials, the most common adverse reaction was eye pain following instillation which was reported in approximately 16% of patients. Eye pain may arise as corneal healing occurs. Other adverse reactions occurring in 1% to 10% of OXERVATE patients included corneal deposits, foreign body sensation, ocular hyperemia, ocular inflammation, photophobia, tearing, and headache.
USE IN SPECIFIC POPULATIONS
Pregnancy
There are no data from the use of OXERVATE in pregnant women to inform any drug associated risks.
Lactation
The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for OXERVATE, and any potential adverse effects on the breastfed infant from OXERVATE.
Pediatric Use
The safety and effectiveness of OXERVATE have been established in the pediatric population. Use of OXERVATE in pediatric patients 2 years of age and older is supported by evidence from adequate and well-controlled trials of OXERVATE in adults with additional safety data in children.
INDICATION
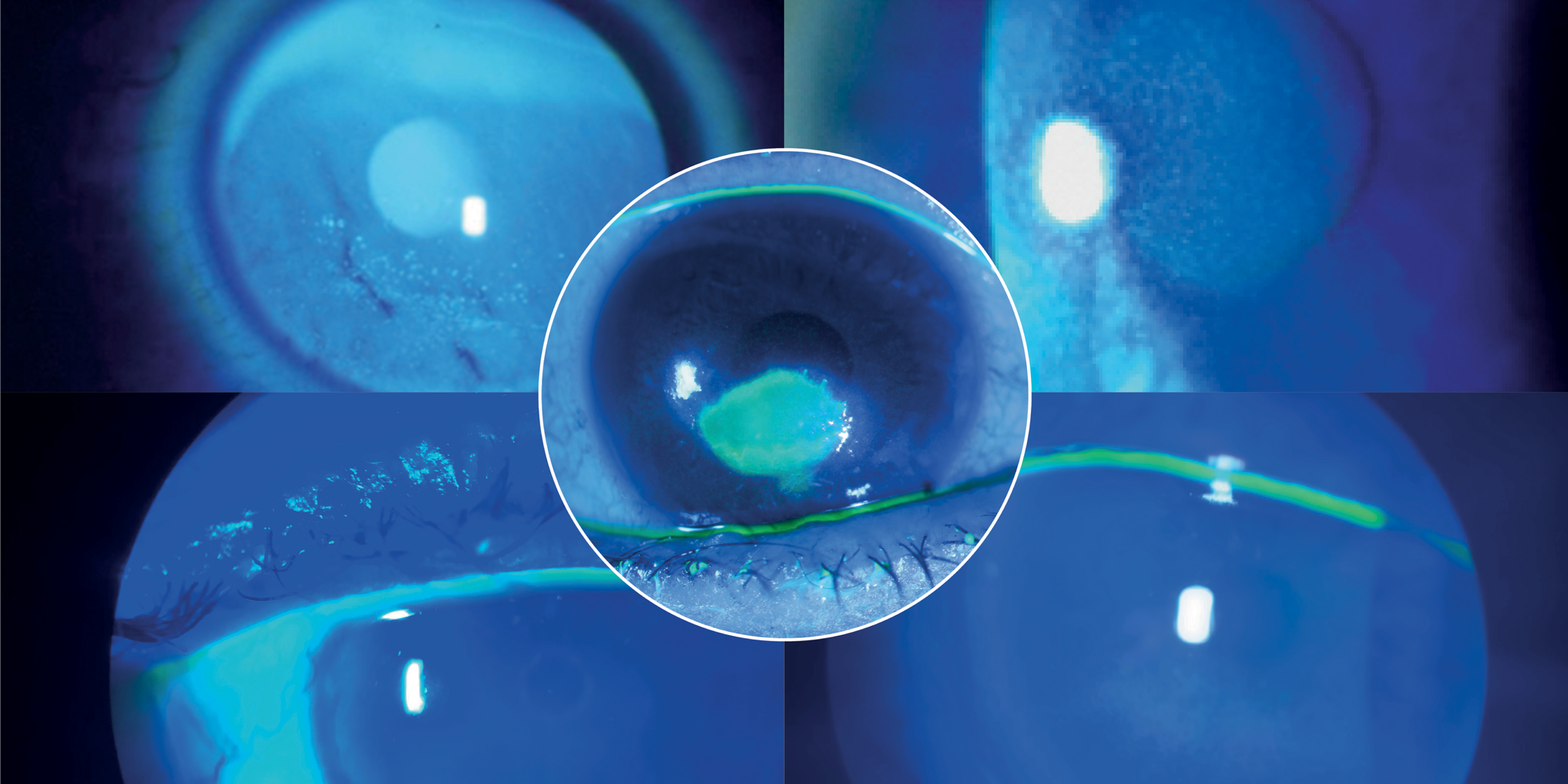
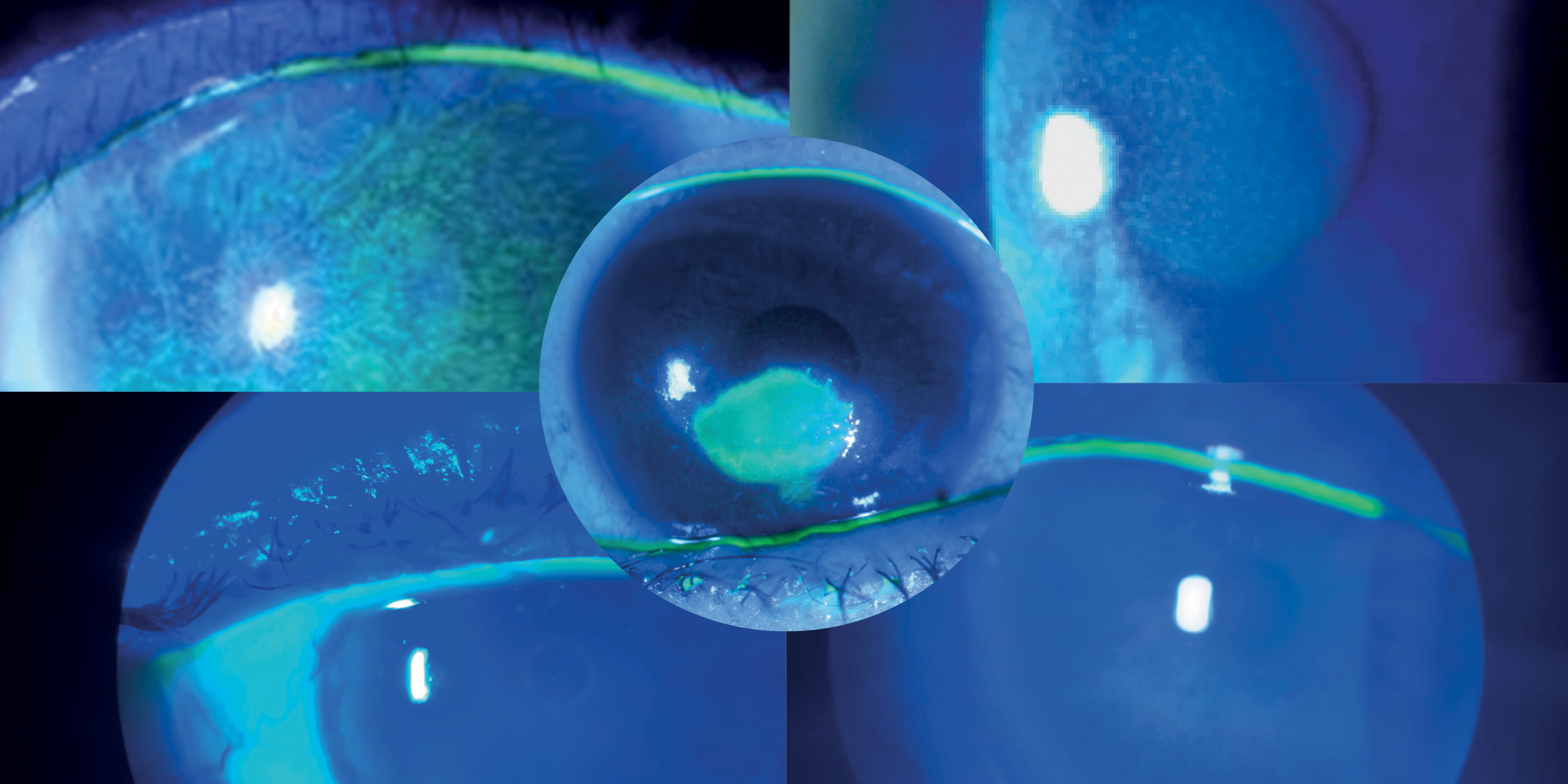
OXERVATE® (cenegermin-bkbj) ophthalmic solution 0.002% (20 mcg/mL) is indicated for the treatment of neurotrophic keratitis.
DOSAGE AND ADMINISTRATION
Instill one drop of OXERVATE in the affected eye(s), 6 times a day at 2-hour intervals for eight weeks.
To report ADVERSE REACTIONS, contact Dompé U.S. Inc. at 1-833-366-7387 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information for OXERVATE.
© 2024 Dompé U.S. Inc. All rights reserved.
US-OXE-2400018 03/24

Take a 5 question quiz to test your knowledge about Neurotrophic Keratitis
