
Espansione Group’s unique, world-leading photobiomodulation (PBM) technology has been developed and patented for medical use in ophthalmology and beyond. After a decade of innovation and advancement in the anterior segment space, the company is applying PBM technology to the posterior pole with treatments for age-related macular degeneration (AMD), specifically in its early stages (i.e., dry AMD).
AMD is a prominent retinal disease that significantly impacts vision, primarily in older adults, leading to irreversible blindness. The disease progresses through early to intermediate stages, eventually reaching an advanced stage characterized by either the dry (85-90 percent of cases) or the neo-vascular form (1). Current treatments are mostly aimed at these later stages, emphasizing the need for early intervention to prevent progression and preserve vision.
At the heart of Espansione’s innovative technology is its patented light-based PBM, also known as Light Modulation® Low-Level Light Therapy (LM® LLLT). This non-invasive treatment uses a spectrum of light wavelengths between 500 and 1000 nm, which is proven to enhance cellular metabolism, increase mitochondrial activity, and, significantly, reduce cell death.
The LightWave I Trial: Illuminating AMD Treatment
The groundbreaking LightWave I trial confirms Espansione's commitment to advancing PBM into novel areas of eye care. Conducted by the company in partnership with nine leading retinal diseases centers across Europe, the trial aimed to evaluate the efficacy of LM® LLLT in preventing the progression of AMD.
The trial used specialized LM® LLLT masks, emitting yellow and red light, with treatment cycles designed to maximize therapeutic benefits – yielding promising results. The study’s lead investigator, Dr. Claudio Iovino (University of Naples Luigi Vanvitelli, Italy) recently presented compelling case reports from the trial describing how AMD patients treated with LM® LLLT showed remarkable improvements, including increased visual acuity and significant reduction in drusen volume, without progressing to atrophic areas. In one case, a 55-year-old male patient diagnosed with non-neovascular AMD saw a staggering rise in visual acuity from 25 to 60 ETDRS letters, accompanied by complete reabsorption of the subretinal fluid, collapse of the pigment epithelial detachment, and no residual retinal atrophy (Figure 1).
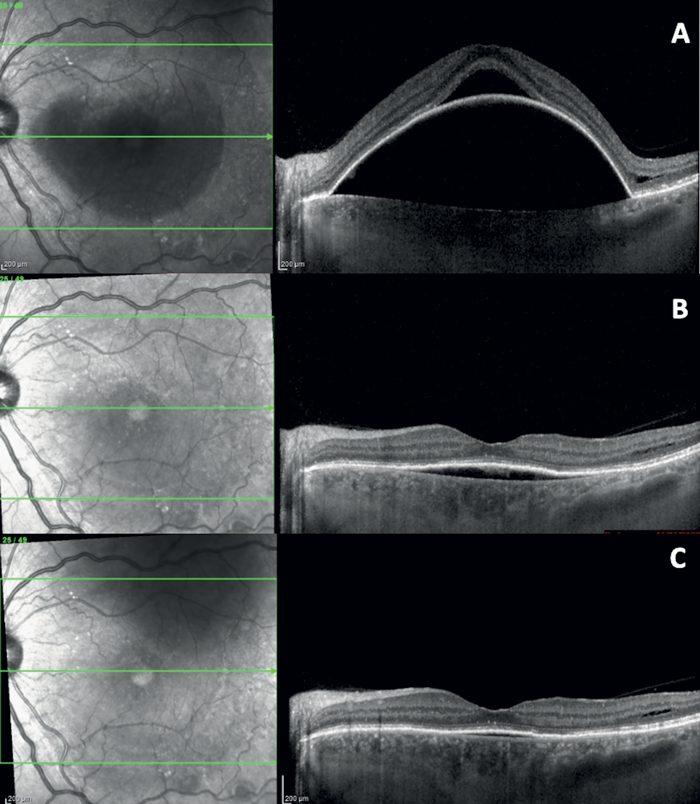
Figure 1. SD-OCT images at baseline (A) exhibit a pronounced pigment epithelial detachment (PED) with subretinal fluid. Image at one-month follow-up (B) reveals a flattening of the PED with the persistence of flat PED accompanied by hyper/hyporeflective material. SD-OCT image at the three-month follow-up (C) demonstrates further flattening of the PED.
As Dr. Iovino pointed out, these outcomes not only underscore the potential of LM® LLLT as a preventive treatment for intermediate AMD, but also highlight its ability to enhance retinal sensitivity and overall visual function. With about 280 million people projected to be affected by AMD by 2040 (1), these results not only pave the way for a new treatment paradigm, but also open avenues for further research, potentially revolutionizing the management of AMD.
Discover the LM® Low-level Light Therapy!
Click here to view the video
Disclaimer: Not all solutions and use cases available in all countries. Every piece of information shown ought to be considered as fact-based evidence deriving from publicly available literature, for the sole purpose of scientific exchange.
References
- NM Schultz et al., “Global Burden of Dry Age-Related Macular Degeneration: A Targeted Literature Review," Clin Ther. 43, 1792 (2021).
