As the world’s population continues to age, degenerative ocular diseases become increasingly common, creating a tremendous burden on healthcare systems. Particularly affecting people over the age of 65, age-related macular degeneration (AMD) accounts for 8.7 percent of all blindness worldwide and is the most common cause of blindness in developed countries. Approximately 85-90 percent of AMD patients have the “dry” form of the disease.
Mitochondrial dysfunction is thought to be a key contributor to the underlying pathology of dry AMD. Mitochondria are essential for energy production, cell function and survival. The visual system is one of the most highly reliant energy systems in the brain and susceptible to functional deficits induced by bioenergetic deficiencies. Up until recently, dry AMD has been a condition without an available therapy (with the exception of guidelines on vitamin supplementation), but this has changed with the introduction of the Valeda® Light Delivery System – the first approved, CE-marked treatment for dry AMD using photobiomodulation (PBM) in Europe. Valeda is in clinical trials and not approved for use in the United States. Valeda provides direct action at the mitochondrial level to improve cellular function. In addition to symptomatic treatment of clinical symptoms, PBM has the potential to provide disease-modifying effects, therefore representing a much-needed treatment option for dry AMD patients, with potential for high impact in the field.
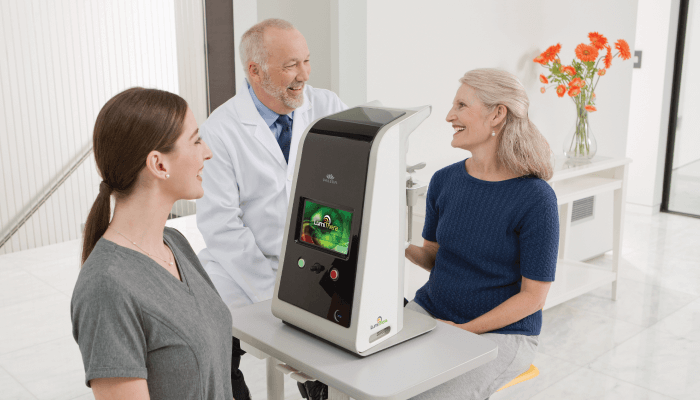
In the EU, Valeda is indicated for the treatment of ocular damage and disease using PBM, including inhibition of inflammatory mediators, edema or drusen deposition, improvement of wound healing following ocular trauma or surgery, and increase in visual acuity and contrast sensitivity in patients with degenerative diseases, such as dry AMD. Valeda was designed for ease of use in the doctor’s office. Indeed, under the doctor’s supervision, trained staff are able to administer the treatment.
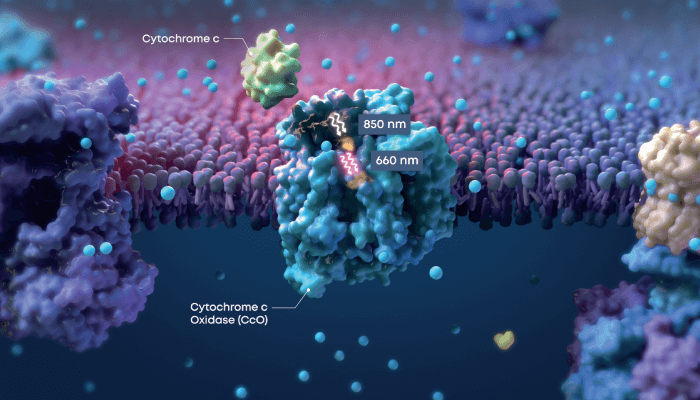
Valeda uses multiwavelength PBM to stimulate critical targets of cellular function, leading to improved energy production within the mitochondria. The mechanism of PBM at the cellular level has been ascribed to the activation of components in the mitochondria, resulting in stabilization of metabolic and cellular function. PBM treatment leads to increased energy production, reduced inflammation, and improved cellular functioning.
Founded in 2013, LumiThera is a commercial-stage medical device company focused on developing treatment solutions using multiwavelength, non-invasive PBM to improve visual function, stop or slow progression of debilitating eye diseases, and prevent vision loss and blindness. PBM has been used in a variety of indications in other medical fields, demonstrating a viable mechanism of action, positive efficacy, and favorable safety profiles. However, its utility in ophthalmology had not been established until now. LumiThera is the first ophthalmic company to develop PBM for the treatment of visual disorders. The company brought together experts in ophthalmology, PBM, and engineering to develop the Valeda Light Delivery System and to create the trial designs to bring Valeda to market and to further research in the ophthalmic field.
Multiple clinical studies demonstrate improvement in clinical (visual acuity and contrast sensitivity), anatomical (drusen reduction), and quality of life outcomes following PBM treatment in dry AMD.
Three clinical studies evaluating the benefit of the LumiThera Valeda Light Delivery System in dry AMD subjects that have been conducted include LIGHTSITE I, LIGHTSITE II, and ELECTROLIGHT. A further US pivotal study, LIGHTSITE III, is fully enrolled and ongoing. The PRIME study (partially funded by NEI) is also underway to evaluate Valeda in diabetic patients with minimal visual impairment but early stage, centralevolving macular edema and diabetic retinopathy.
LumiThera’s initial clinical trials were focused on dry AMD. Other ophthalmic conditions present with overlapping pathology, which indicates a high potential for translation to other ocular indications. Indeed, LumiThera has multiple ongoing studies to evaluate Valeda treatment in additional disease states.
