
Prior to the advent of minimally invasive glaucoma surgery (MIGS), first-line glaucoma treatment was generally medical therapy – with one or more topical ocular hypotensive drugs. When medical treatment failed, or when adherence to medical therapy was inadequate, surgical intervention was considered. In the past, the “gold-standard” surgical procedure has been trabeculectomy, an invasive procedure that creates a fistula between the anterior chamber and the subconjunctival space. Evidently, treatment norms have been evolving over the past 10–15 years. In particular, the introduction of MIGS devices and procedures has lowered the threshold for surgical intervention to the point where it is now an accepted early disease treatment option.
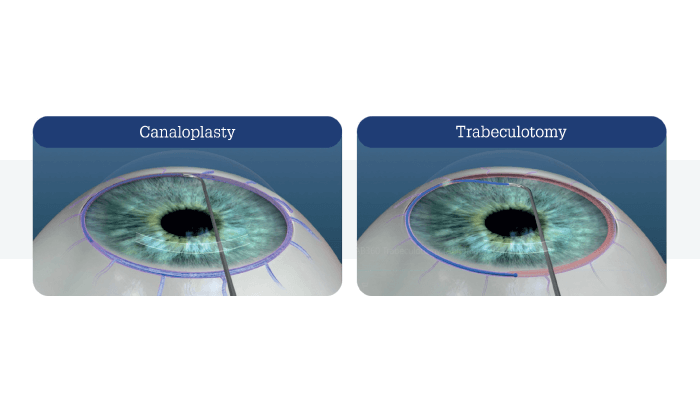
The OMNI Surgical System from Sight Sciences is a relatively new addition to the MIGS armamentarium. The system performs both 360° canaloplasty and trabeculotomy in a single surgical session, using a single implant-free device and through a single small (<2 mm) clear corneal incision. The device is CE-marked for canaloplasty and trabeculotomy to reduce intraocular pressure in adult patients with open-angle glaucoma. Notably, the OMNI Surgical System allows surgeons to address outflow resistance, both proximally (juxtacanalicular trabecular meshwork and inner wall of Schlemm’s canal), and distally (Schlemm’s canal and the collector channels).
What makes the OMNI Surgical System unique? It is the only device that combines two accepted and well-established mechanisms to address outflow resistance. This translates into two critical physiological and clinical advantages: firstly, it can comprehensively address up to 360 degrees of the diseased conventional outflow pathway in an implant-free manner. (Trabecular-bypass stents address a much smaller segment.) Secondly, the OMNI Surgical System tackles all three points of resistance in the conventional outflow pathway circumferentially, which currently available stent implants cannot achieve.
The OMNI Surgical System is rapidly gaining acceptance in the field of glaucoma surgery for adult populations. Since initial commercialization in early 2018, over 1,000 surgeons globally have been trained and many use the OMNI Surgical System regularly to care for their glaucoma patients. To date, over 50,000 adult glaucoma patients have been treated with the OMNI Surgical System.
The OMNI Surgical System’s indication for use broadly covers the reduction of intraocular pressure for all adult patients with OAG – without limitation to severity of disease (mild, moderate, and advanced) or lens status (phakic and pseudophakic). The broad indication represents a significant step forward in identifying which MIGS procedures are appropriate for certain indications rather than as a “do no harm” add-on to cataract surgery.
Sight Sciences is a growth-stage medical device company transforming the two fastest growing segments in ophthalmology and optometry, glaucoma and dry eye disease. Founded in 2011, the company has already made a name for itself with intelligently designed and engineered products that push the boundaries of what’s possible.
IMPORTANT PRODUCT INFORMATION: The OMNI™ Surgical System is indicated for the catheterization and transluminal viscodilation of Schlemm’s canal and the cutting of trabecular meshwork to reduce intraocular pressure in adult patients with open-angle glaucoma.
For important safety information including contraindications, warnings, precautions and adverse events, please visit omnisurgical.com.
CE 2797
12/21 OM-2029-OUS.v1
