About half of all patients with diabetic retinopathy (DR) go on to develop DME. But as those patients will know, current treatment options can present a significant burden, requiring either regular injections or surgery. Moreover, there is a lack of suitable treatments for early DME patients – and a low benefit-to-risk ratio for mild DME sufferers.
So why aren’t there more treatment options for these patients? Well, one of the more obvious options – conventional eye drops – pose a number of hurdles for drug manufacturers, including limited solubility of drugs in such formulations, rapid clearance from the eye, and difficulty delivering drugs to the retina.
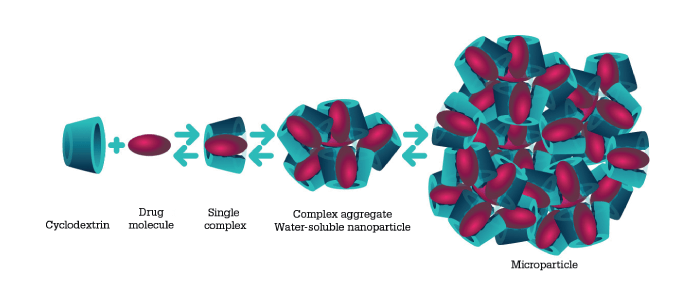
Now, a novel formulation approach could help overcome current limitations: solubilizing nanoparticle (SNP) technology.
These complex nanoparticles, which benefit from the unique characteristics of a compound called cyclodextrin, enhance drug solubility in aqueous tear fluid. Typically, the residence time for an eye drop is between two and four minutes – but SNP technology increases residence time to 8 to 12 hours; not only that, it also allows a much higher drug concentration. Moreover, the drug–cyclodextrin complexes are able to more easily penetrate the mucous layer of the eye, enabling sustained drug release and increased bioavailability.
In a recent Phase II study, the efficacy of Oculis’ OCS-01 – based on SNP technology – was tested for the treatment of DME. The results will be presented at the Angiogenesis, Exudation, and Degeneration 2020 meeting, taking place on February 8, 2020 in Miami, USA. If positive, it could represent a step forward in DME treatment.
“Recently, a lot of the research focus has been on developing injectables, but we really believe topicals are important for DME,” says Riad Sherif, CEO of Oculis. “Essentially, the technology gives ophthalmologists another tool in their toolbox, and gives patients another treatment option. We believe that by creating a topical treatment for DME, we can really help doctors to act quickly and improve the vision of patients.”
