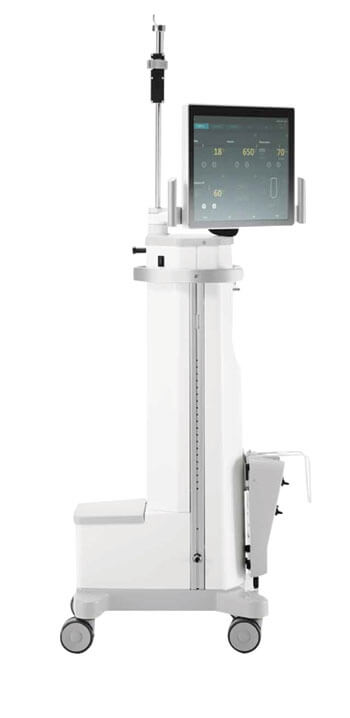
Founded in 2014, THIS AG brings together experts with proven and practical experience in medical device R&D and commercialization. Such backgrounds imply an appreciation of the importance of market research – and indeed, THIS AG’s product development program is informed by three broad requirements in the field of phacoemulsification instruments: mobility, simplicity and safety. Together, improvements in these areas can enhance efficiency before, during and after the phaco procedure, and benefit both the patient and the surgical team.
Accordingly, THIS AG has developed the SWISS OPHTHALMOLOGY INNOVATION (SOPHI) brand. The advance is based on three pillars of innovation which, in sum, address the above market requirements. Firstly, the exceptional mobility and flexibility of SOPHI enable the surgical team to adapt the new system to their needs on a day-to-day basis. Secondly, SOPHI’s highly effective sinus phaco function, combined with multiple energy saving modes, reduces the amount of energy delivered to the eye. And thirdly, the unique triple-pump fluidics system integral to SOPHI enhances overall efficiency and safety throughout the entire phaco procedure.
The three advantages arise from a suite of technical innovations built into the SOPHI system. Novel features include: a communication system, incorporating a simple touchscreen graphical user interface with text-to-speech functionality and color-changing LEDs, as well as wireless data communication and WiFi-enabled video inlay. In terms of advanced fluidics management, SOPHI incorporates three coordinated pumps: two peristaltic devices (for improved aspiration and for unique active infusion control (IOP control)) and a clean Venturi pump, which provides a mechanical barrier between the patient fluidics and the device, thereby preventing contamination. The pumps are supported by an infusion level guard, an automatic cassette slot, and individual sensors within the cassette monitor surge detection, flow measurement within the Venturi, water/air detection – and monitoring of the waste bag (a warning is triggered when full).
Finally, SOPHI offers various electro-mechanical enhancements to make the surgeon’s life easier, including battery-supplied operation; an internal compressor; an easy-move foot pedal with inductive charge; a rotatable screen and tray allowing unparalleled flexibility of placement; and an easy-brake system for comfortable positioning.
The overall result? A high-end device that sets new standards in the field of phacoemulsification instruments – as CEO Thomas Köppel puts it: “The SOPHI brand itself is a commitment to innovation.” SOPHI has already reached the final stage prior to CE marking, and launch is anticipated for 2019, reflecting THIS AG’s ability to rapidly and efficiently re-think the future of phaco instrumentation. THIS AG’s philosophy of innovative device development in areas that can be improved is a mode of thinking that retains a focus on individuals, including employees, partners, customers and, not least, patients.
And this is just the beginning. Köppel asserts that SOPHI is only the first milestone in THIS AG’s journey, and states that the ophthalmology community should expect further advances from the company. So if you need gentler, safer treatments for your patients, and more ergonomic, better-optimized systems for your surgery, think seriously about THIS.
