If we want to crack a hazelnut, we don’t reach for a sledgehammer. Likewise, effective ophthalmic surgery requires precise application of subtle force. Fortunately, Ziemer has hard-wired subtlety and precision into its Z8 system: high-frequency, low-energy femtosecond laser pulses, guided by intra-operative OCT, bring new delicacy and accuracy to the most difficult procedures. How are surgeons applying the new femtolaser system in their daily practice? To find out, Ziemer convened a symposium at the 36th ESCRS in Vienna (September 22, 2018).
Featuring:
Chair: Theo Seiler, MD, PhD, Professor, IROC AG, Zurich, Switzerland
Shady Awwad, MD, Associate Professor of Ophthalmology, Head Cornea and Refractive Surgery Division, American University of Beirut Medical Centre, Beirut, Lebanon
Soon-Phaik Chee, MD, Professor, National University of Singapore and Duke-National University of Singapore Graduate Medical School, Singapore
Jod Mehta, MD, Associate Professor, Head of the Corneal and External Eye Disease Service, Singapore National Eye Center and Duke-National University of Singapore Graduate Medical School, Singapore
Gerald Schmidinger, MD, Surgical Senior Physician at the Department of Ophthalmology, Head of the cornea bank, head of the outpatient clinic for corneal diseases, Medical University of Vienna, Austria
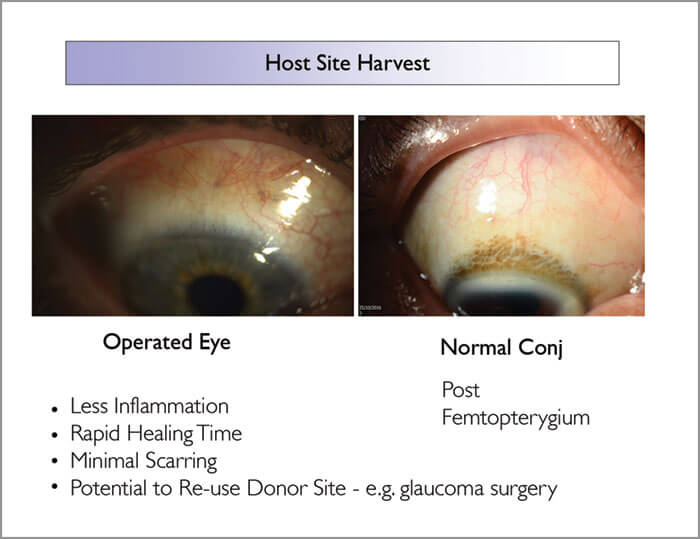
Femto-Pterygium, a new indication for the femtolaser, is made possible by two critical features of the Z8: the high numerical aperture and the articulating hand piece. The former permits the ability to cut through opaque tissue such as the conjunctiva; the latter allows the surgeon to excise a graft from the superior bulbar surface by rotating the eye down to complete the surgery. In previous work, we showed that Femtosecond Laser-Assisted Pterygium Surgery (FLAPS) worked very well in porcine eyes (1). Subsequently, our first-in-man FLAPS trial (2) reported similarly encouraging results: rapid creation of ultrathin grafts, high case-to-case reproducibility, good cosmesis, and minimal scarring at both bulbar surface and graft site.
These results are partly due to the reproducibility of FLAPS: standard deviation and inter-surgeon differences are lower for 60 than for 100-micron grafts. The thickness of FLAPS is also significant: by restricting CAG thickness to 60 microns, we stay within the conjunctiva epithelium, and thereby avoid disrupting Tenon’s capsule. As conjunctiva thickness changes with age, 60 microns will encompass 98–99 percent of individuals (3); shallow grafts help minimize scarring in older patients. Furthermore, the gentle, low energy Z8 causes little inflammation; hence faster and better healing, and what we see clinically (Figure 1) is consistent with OCT angiography data showing rapid revascularization of graft sites (Figure 2). Moreover, scarless healing allows us to re-use the donor site if needed, for example, in glaucoma surgery.
We’ve now compared 30 femto-pterygium cases with 120 standard pterygium surgeries – and the results are striking (Sidebar 1): i) a mean graft thickness of 74 microns with a graft-lift time of about 5 seconds, ii) time savings of about 5-10 minutes per procedure, and iii) significant reductions in pain score and recurrence rates. The instrument also brings reassuring flexibility to the OR; if the patient moves during conjunctival incision, we simply reprogram the laser, re-dock it on the conjunctiva, and proceed as if it were a virgin cut – with the same outcome. In summary, FLAPS provides faster surgery, less scarring, and less post-operative pain than standard pterygium surgery. Overall, it standardizes procedures, irrespective of surgeon experience.
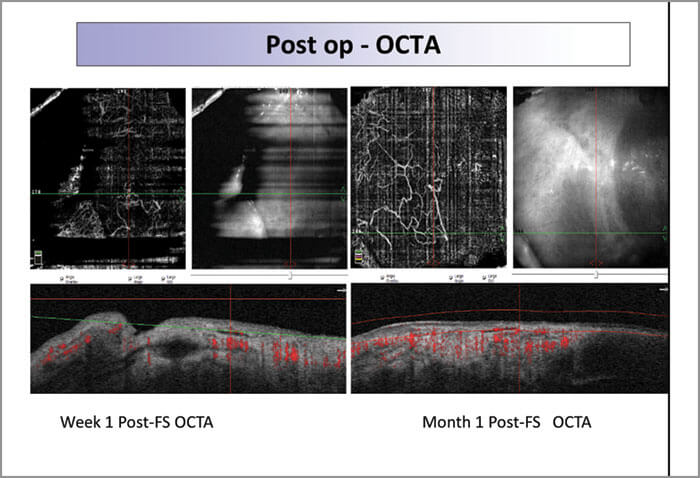
Femto-Pterygium forms precise, planar cuts, leaves conjunctival blood vessels still patent and functional, and reduces inter-surgeon variability in cut depth.
- 30 prospective patients
- Mean defect size: 5.8 x 9.1 mm
- Pre-set size: 7 x 10 mm
- Actual graft size: 6 x 9.7 mm
- Desired graft thickness: 60 µm
- Actual graft thickness: C 74.2 µm, P 74.8/75.3 µm
- Time to lift graft: 4.9 seconds
- No recurrences after one-year follow up
When faced with a very dense nucleus and a thick, leathery posterior plate, one’s instinct is to use more energy. In fact, it’s possible to manage dense cataracts with low energy levels – by applying high-frequency pulses of nanojoule energy. Such an approach also brings a number of advantages: smoother capsulotomies, lower risk of tag formation or anterior capsule rip, cleaner fragmentation, and lower levels of bubble formation.
Correct technique, however, is essential to getting the most out of the low-energy approach. In particular, we should modulate our procedure according to cataract density. For example, with a moderately dense cataract I would cut eight segments, and keep the offset at 600-700 microns. With denser cataracts, I might cut 16 segments, which makes it simpler to separate the nucleus into smaller pieces to allow the fragments to be easily mobilized from the capsular bag and emulsified. This involves lateral separation of the uncut posterior and peripheral portion of the nucleus..
Similarly, fragmentation of a dense posterior polar cataract is made much easier by pre-cutting it into eight segments without adjusting the posterior offset. This helps the surgeon to remove the fragments in a stepwise manner without rotation. You can apply the same technique even to a dense brown cataract; you simply need to impale the nucleus deeply and then separate the fragments with the second instrument.
In white intumescent cataracts, I find it helpful to use capsular dyes to show up incomplete capsulotomies or areas of capsular tags or sub-capsular fibrosis. Their presence requires one to increase the capsulotomy energy to ensure you cut through any fibrotic strands. Often, dense cataracts are accompanied by weak zonules; if so, I use a capsular bag hook followed by a capsular tension ring, as this approach prevents the vitreous from coming forward.
So the Z8 makes even very dense cataracts relatively simple – the laser does most of the segmentation, and pre-cutting the nucleus helps the surgeon manage complicated cases while preserving both cornea and posterior capsule. The lower energy results in fewer bubbles produced, which both improves visibility and helps surgeons to avoid bubble-induced distension of the posterior capsule.
In summary, a variety of dense cataracts can be more efficiently and safely managed with femtosecond laser-assisted cataract surgery (FLACS) than with manual cataract surgery, but optimal outcomes require adjustment of parameters and technique.
The advantages of Femto-DALK include:
- better side-cut geometry
- more accurate cut-depths (for side-cut and for lamellar dissection)
- better trephination centration
- less risk of donor cell damage
- OCT feature, which allows intra-operative adjustments of the laser
Deep anterior lamellar keratoplasty (DALK) is as difficult, as it is beneficial. Can the femtolaser make it more accessible?
In my experience, a femtosecond laser is superior to the manual approach; it achieves better side-cut geometry, more accurate cut-depths (both for the side-cut and for lamellar dissection) and better trephination centration during applanation (the OCT feature allows intra-operative adjustment of the laser). Furthermore, femto-DALK causes less donor cell damage than manual techniques.
Applying femto-DALK to an eye with advanced keratoconus (www.femtoldv.com/femto-dalk) illustrates how intra-operative OCT enables adjustment of cut depth and maintenance of a safe 150-micron margin. This approach clearly reduces the risk of anterior chamber penetration.
But perhaps the most advantageous aspect of femto-DALK is its ability to form guiding tunnels of precise depth to direct the DALK cannula. This enables deep cuts to be made safely within 50-80 microns of the endothelium, with a high degree of precision (actual distances and intended distances to endothelium are close, with low standard deviations, in both human and porcine eyes (7)).
Clinical outcomes are promising: one group achieved a Type I bubble in each of 14 cases (7), with zero intraoperative laser-related complications (guiding tunnel set at 50 microns from the endothelium, C 74.2mm diameter trephination); my own group achieved Type I bubbles in 92 percent of 36 eyes (8-8.5 mm diameter trephination, guiding tunnel set at 80 microns from endothelium).
In conclusion, the Ziemer Femto LDV Z8 is transforming DALK surgery. Intra-operative OCT replaces subjective judgments with unambiguous intraoperative measurements, simplifies and standardizes the procedure, and makes the big bubble step more reproducible. Inexperienced surgeons wishing to participate in DALK will find the Z8 invaluable.
Conclusions
The symposium participants appeared to reach consensus: the enhanced precision and reduced energy of Z8 are transforming established techniques and enabling new procedures. Intra-operative visualization of the entire cornea permits confident positioning of keratoplasty incisions for optimal safety and outcomes. Similarly, OCT-directed positioning of guiding tunnels close to Descemet’s membrane increases confidence, making DALK far more accessible.
Furthermore, the uniquely low energy delivered by Z8 permits delicate procedures, such as ring insertion, without incurring costs related to bubble formation or bridge generation, and also results in rapid vascularization and straightforward healing. Kindness is not the same as weakness, however: the low energy laser can help fragment the densest cataracts, making difficult cases routine.
Thus, with Z8, the surgeon gets not different limitations, but extended capabilities. The instrumentation enables new procedures, such as femto-pterygium, while transforming older procedures, such as DALK and post-phaco LASIK. And that is the real power of low energy.
Shady Awwad
Corneal rings are essential in keratoconus management; they flatten the central cornea and steepen axis 90 degrees away from the incision. Depth is a key aspect of intrastromal ring segments success and safety. Shallow tunnels can lead to anterior stromal melting and neovessels. Deep tunnels can result in Descemet’s perforation due to gas bubbles or due to direct impingement of the ring segments. Cross-linked corneas may also cause problems as they might look deceptively thick on Scheimpflug pachymetry. Finally, advanced keratoconus can raise centration problems, which may be difficult to resolve intraoperatively without real-time pachymetry guidance. Such challenges may cause surgeons to avoid all except the simplest cases of keratoconus.
Now, the Z8 is transforming keratoconus management. Key features include intra-operative OCT and a very low energy laser. The latter enables precise, shallow cuts with minimal collateral damage, and without leaving bridges. The former allows the surgeon to check the depth of the planned tunnel intraoperatively in real time, make necessary adjustments, and then check the actual depth after tunnel creation. Thus, the Z8 empowers surgeons to confidently accept the most challenging corneas. It provides unparalleled safety together with extraordinary precision and reliability; the outcomes speak for themselves. I believe it is the ultimate keratoconus platform.
Theo Seiler
Multifocal IOLs have intrinsic limitations; for example, glare and halo become significant where residual astigmatism is 0.5 diopters or less. And that’s why many multifocal IOL recipients are unhappy. Our own data (108 patients) show that 40 percent of patients are dissatisfied with their vision (their mean satisfaction score, on scale of 1-4, was 2.1) for reasons including astigmatism, myopia, hyperopia and higher order aberrations.
Correcting these issues with Z8-mediated LASIK is ideal, as it allows me to select which aberrations to address; for example, so as to modulate only those that interfere with near vision. And the patients appreciate it: one month after surgery, the mean satisfaction score increased from 2.1 to 3.6, and 90 percent of previously dissatisfied patients said they would choose the procedure again.
Therefore, “giving” LASIK to every multifocal IOL recipient patient who requires it should increase overall patient satisfaction. Bundling LASIK with multifocal IOL implantation, however, has economic implications. If we assume that femtoLASIK costs $1300, and that 26 percent of multifocal IOL patients subsequently require corrective LASIK, we can calculate the extra cost per multifocal IOL procedure: 1300 x 0.26 = $338. And that gives us an economic footing from which to offer patients a package that includes LASIK “for free,” if it is required to correct residual refractive errors.

References
- M Fuest, et al., “Femtosecond laser-assisted conjunctival autograft preparation for pterygium surgery”, Ocul Surf, 15, 211-217 (2017). PMID: 27919798. M Fuest, et al., “Femtosecond laser-assisted pterygium surgery”, Cornea, 36, 889-892 (2017). PMID: 28489723. X Zhang, et al., “Bulbar conjunctival thickness measurements with optical coherence tomography in healthy Chinese subjects”, Invest Ophthalmol Vis Sci, 54, 4705-9 (2013). PMID: 23744999. JS Lee, et al., “Efficacy and safety of large conjunctival autograft for recurrent pterygium”, Korean J Ophthalmol,31, 469-478 (2017). PMID: 29230976. M Keane, et al., “Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus”, Cochrane Database Syst Rev, 2014 doi: 10.1002/14651858.CD009700.pub2 G Chen, et al., “Deep anterior lamellar keratoplasty versus penetrating keratoplasty: a meta-analysis of randomized controlled trials”, Cornea, 35, 169-74 (2016). PMID: 26583281. Liu, et al., “Femtosecond laser-assisted deep
