For nearly 40 years, the standard treatment for proliferative diabetic retinopathy (PDR) has been panretinal photocoagulation (PRP). It works (partly through reducing VEGF), but at a cost: it risks permanent visual field loss, DME exacerbation – and even with timely PRP treatment, 1 in 20 eyes still go on to develop severe vision loss. As retinal neovascularization is a central component of PDR, might the mighty anti-VEGF agents that have been so successful in treating other retinal neovascular disease supplant the venerable PRP? In 2015, the DRCR.net published the results of Protocol S (1), which compared the outcomes of patients with high-risk PDR (with and without macular edema) treated with PRP or ranibizumab 0.5 mg. After two years of follow-up, the conclusion was that “treatment with ranibizumab resulted in visual acuity that was noninferior to […] PRP” and that “ranibizumab may be a reasonable treatment alternative, at least through 2 years.”
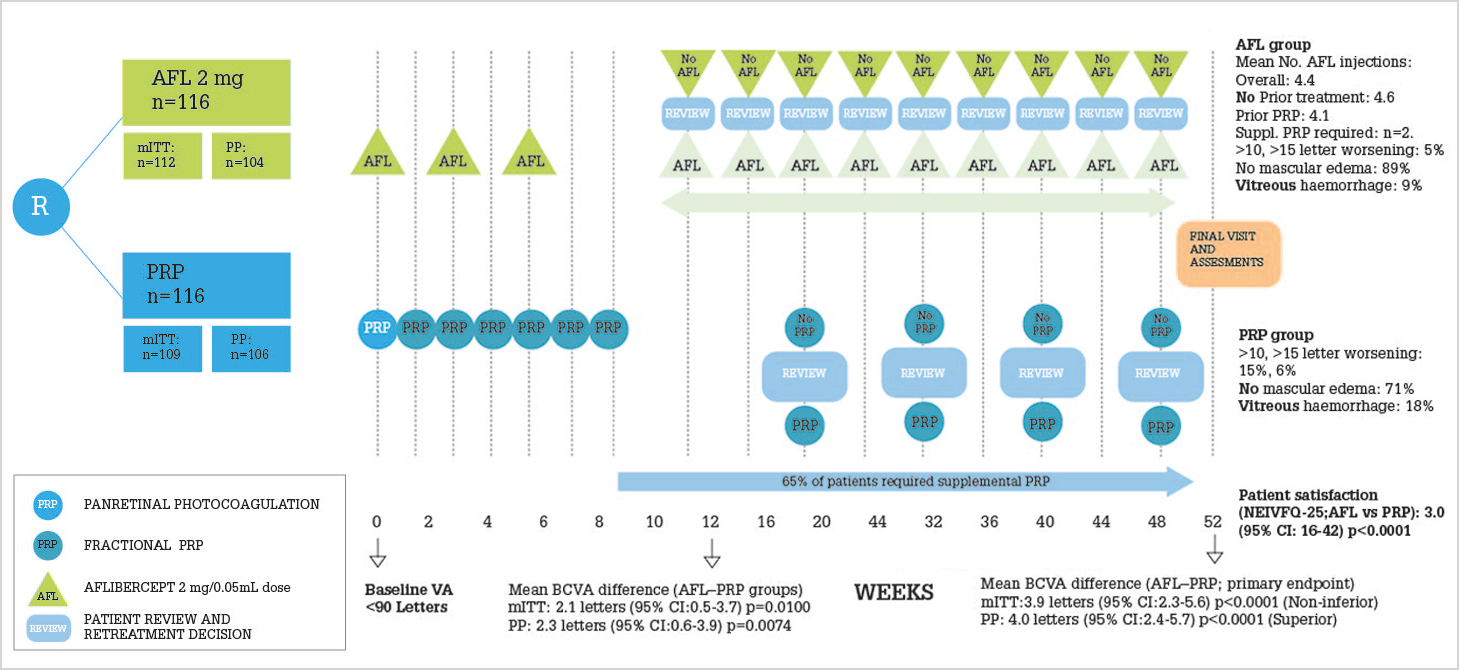
But what about aflibercept? CLARITY (2) was a multicenter Phase IIb non-inferiority trial that compared aflibercept 2 mg with PRP over a one-year period. The study design and key results are illustrated in Figure 1, but the essence is this: patients with PDR, treated with aflibercept, displayed superior visual acuity after one year compared with those treated with PRP. The study’s chief investigator, Sobha Sivaprasad, says: “This study is the first to show superior visual outcomes with an anti-VEGF agent, when compared to PRP, in proliferative diabetic retinopathy without baseline macular edema. These are significant findings for an eye condition that has been treated with PRP as standard-of-care for the past 40 years to demonstrate that aflibercept could potentially be adopted as an alternative treatment option, in the first year, in compliant PDR patients in the future.” She also notes further avenues for investigation: “The three initial doses may represent over-treatment, and spreading a few doses evenly across the year may offer a cost-effective alternative to PRP.”
References
- JG Gross et al., “Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy”, JAMA, 314, 2137–2146 (2015). PMID: 26565927. S Sivaprasad et al., “Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial”, Lancet, Epub ahead of print (2017). PMID: 28494920.
