The question of whether or not the ocular surface harbors resident microbiota has been under debate for a long time; microbial organisms can be found on the surface of the eye, but they could have arrived there from the surrounding environment. Now there’s an answer to this long standing deliberation; a resident ocular biome does exist – and it helps defend the ocular surface from pathogens by tuning local immunity. “Originally, we were collaboratively studying Muckle-Wells disease, a condition which results in generalized inflammatory syndrome and is accompanied by conjunctivitis, in mice,” says Rachel Caspi, of the National Eye Institute and lead author on the corresponding paper (1). Interested in the conjunctivitis aspect, the team hypothesized that these individuals (and the mice carrying the mutation) may be responding abnormally to normal stimuli from the environment. Caspi says, “We’d also observed that immunologically deficient mice at our facility developed conjunctivitis as they aged. Microorganisms were one possibility so we started to examine healthy and mutant mice, and the rest is history…”
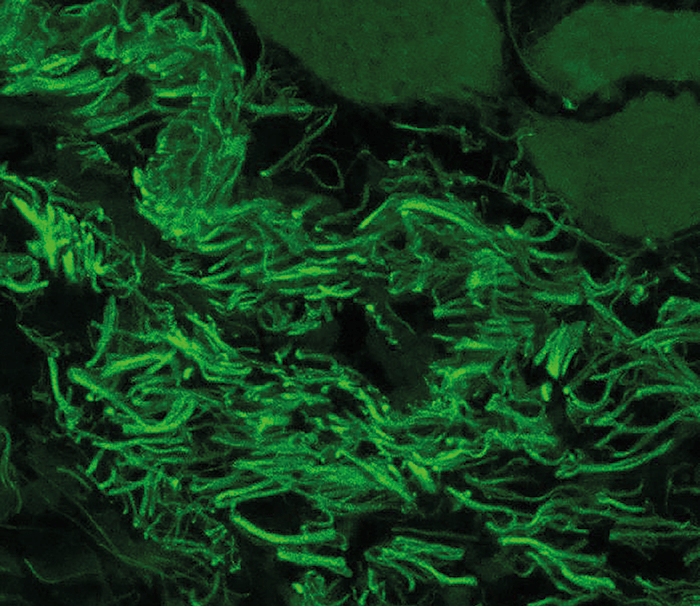
The team identified that Corynebacterium mastiditis – a known skin commensal – formed stable colonies on the conjunctiva of wild-type mice bred at their institute, as well as mice provided by a collaborator at Washington University (Figure 1); C. mastiditis was not found on the ocular surface of commercially sourced mice. Caspi comments, “We expected to find stimuli from the microbial world on the ocular surface, but didn’t expect them to be ‘permanent’ residents on the eye.”
But how did they demonstrate the bacteria were resident and not simply being reinoculated into the eye from the air, mouth, nose or skin? “It is very difficult to show this, but we were lucky that the mice purchased from commercial vendors lacked this bacterium, and that it could not be transferred through co-housing with mice harboring the bacteria,” explains Caspi. True commensalism was demonstrated through detecting C. mastiditis on vendor mice conjunctiva for as long as five weeks after inoculation. “This gives us a measure of confidence that C. mastiditis cultured from the eyes of vendor mice had taken up residence and wasn’t the result of continuous reinoculation.” Interestingly, the team found that the conjunctival commensal helped protect the eye from infection: it induced secretion of interleukin-17 from γδ T cells, which consequently drove neutrophil recruitment and secretion of antimicrobial agents into the tears. They also discovered that mice treated with gentamicin and vendor mice lacking C. mastidits showed increased susceptibility to fungal (Candida albicans) and bacterial (Pseudomonas aeruginosa) infections, respectively. The team believe their findings have implications for the use of antibiotics to treat conjunctivitis, which Caspi thinks may bring more harm than good. This suggests that current antibiotics should be used for the shortest period possible. Looking to the future, Caspi says, “Probiotics based on bacterial extracts could be developed for the eye to stimulate natural immunity on the ocular surface and promote the body’s own defenses, reducing the need for antibiotics.”
References
- AJ St Leger et al., “An ocular commensal protects against corneal infection by driving an interleukin-17 response from mucosal γδ T cells”, Immunity, 47, 148–158 (2017). PMID: 28709803.
