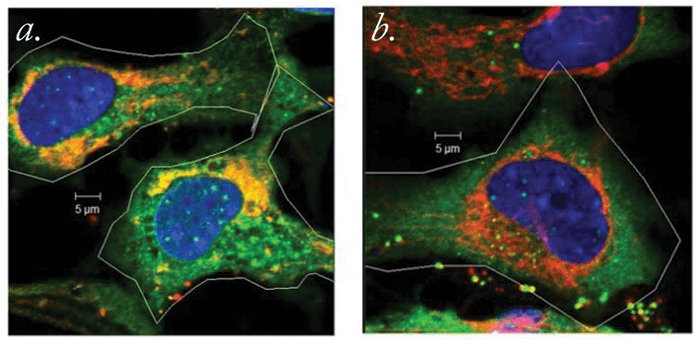
Parkin is an interesting protein. Encoded in humans by the PARK2 gene, it is implicated in several disease states, including Parkinson’s disease. How PARK2 mutations lead to dopaminergic cell death and early Parkinsonian symptoms isn’t clear, though – it appears to play a role in the degradation of free-radical-damaged mitochondria, but what’s now clear is that parkin also plays a central role in keeping the lens… clear.
Intrigued by the protein’s potential role in lens opacity, researchers from the Charles E Schmidt College of Medicine at Florida Atlantic University decided to delve deeper. They performed cell culture experiments in which lens epithelial cells (LECs) expressing either normal or mutated forms of the PARK2 gene were assessed. What they found was this: PARK2 is expressed when LECs are exposed to cataract-causing, free radical-generating environmental insults (in this case, oxidative stress caused by hydrogen peroxide exposure). Parkin removes damaged mitochondria (see Figure 1), and by doing so, helps prevent the formation of free radicals in LECs. This increases the ability of the LECs to survive free radical formation, and presumably, the reactive oxygen species-mediated aging.
“Our findings suggest that parkin plays a direct role in the prevention of oxidative stress through its ability to maintain cellular mitochondrial populations, and that the gene encoding parkin is induced by environmental damage,” says Marc Kantorow, lead author of the associated paper (1). What could this mean? “Drugs or genetic methods that increase parkin levels and function could prove effective in preventing cataracts and other age-related degenerative diseases, including neurological conditions like Parkinson’s disease,” he explains. According to Kantorow, the team now plans to “establish how parkin regulates the growth and development of the lens by controlling mitochondrial populations that are required for lens cell growth.” He adds, “We want to identify the genetic mechanisms that regulate the production of parkin in cells and see if they can be manipulated to increase parkin levels, thereby increasing cell survival to prevent disease.”
References
- L Brennan et al., “Parkin elimination of mitochondria is important for maintenance of lens epithelial cell ROS levels and survival upon oxidative stress exposure”, Biochim Biophys Acta, 1863, 21–32 (2017). PMID: 27702626.
