Twenty-five years old is a good age to be. Any older and your hearing starts to decline; the loss may be barely detectable at that stage – but it’s only going in one direction. By the start of your fourth decade, your bone and skeletal muscle mass starts to decline, and by your mid-forties, a number of ocular diseases start to manifest: incipient cataract, slight drusen deposits, a small raise in IOP... and presbyopia. Some people experience it in their forties, others in their fifties. It’s most definitely age-related, and for now, almost certainly inescapable. The progressive loss of accommodation (Table 1) is the big issue with presbyopia because it brings with it the inability to focus on near visual tasks (1). In developed countries, that’s easily dealt with: you wear spectacles. But having to wear them for near tasks can be a pain, and we all know that there’s a great appetite for being “spectacle-free” – it’s what drove the LASIK boom of the early 2000s. Glasses cost money, prescriptions change, they can get broken and lost, they need to be cleaned and cared for, and you might not like how you look wearing them – all valid complaints. But if you are in a developing country, presbyopia is more of a problem. If your livelihood or survival requires the use of near vision and you don’t have access to spectacles, you’re in trouble. Clearly, solving the presbyopia problem has the potential to transform many lives, which is why presbyopia correction is often referred to as the “Holy Grail of refractive surgery” (2).
Over the years, many surgical strategies have been developed in an attempt to correct presbyopia, but none have truly solved the problem, with each having risks and limitations, and all resulting in some compromise in visual function. But what about a pharmacological approach to reduce the impact of presbyopia?
Appreciating the anatomy
To understand how drugs might be used to treat presbyopia, it’s worthwhile considering the main anatomical units involved in accommodation: the ciliary muscle, lens and zonular fibers. The ciliary muscle is predominantly under parasympathetic control, with sympathetic innervation playing a minor role in relaxation only (by inhibiting accommodation). The contraction of the ciliary muscle alters the shape and position of the lens, thereby invoking the accommodative capacity. Notably, the ciliary muscle also contains muscarinic-3 acetylcholine receptors (M3). It’s important to note that the iris is also under heavy influence of the parasympathetic system – cholinergic stimulation of the M3 receptors on the iris sphincter muscle causes miosis. Conversely, the iris dilator muscle is sympathetically innervated and contains α-adrenergic receptors. If these receptors are antagonized, it allows the parasympathetically innervated pupillary sphincter to predominate (as it is unopposed), resulting in miosis.Depth of focus: the pinhole effect
Most pharmacological approaches for the treatment of presbyopia are based on reducing the aperture of the pupil. Small aperture optics have long been known to increase near visual acuity (VA) by increasing the depth of focus. Peripheral light waves are most distorted by refractive error: by blocking these and allowing only the most central rays of light to reach the retina, this results in not only clearer vision, but also an increase in the depth of field of clear vision. It’s this principle that underpins the surgical approaches of AcuFocus’ small aperture corneal inlays and intraocular lenses (IOLs). Their Kamra corneal inlay features a central 1.6 mm aperture (a size AcuFocus claim achieves an expanded depth of focus without significant visual degradation) and their IC-8 IOL contains a 1.36 mm central aperture and is implanted in the non-dominant eye – and has shown promising early results. Clearly, for a pharmacological option to be successful it must create a similar and long-lasting effect on pupil size (3). However, manipulating pupil size is not without undesired consequences. A constricted pupil (understandably) decreases vision at night – less light enters the eye, and diffraction at very small pupil sizes can degrade overall vision quality. For elderly patients in particular, decreasing the amount of light falling on the retina worsens vision.Understanding accommodation
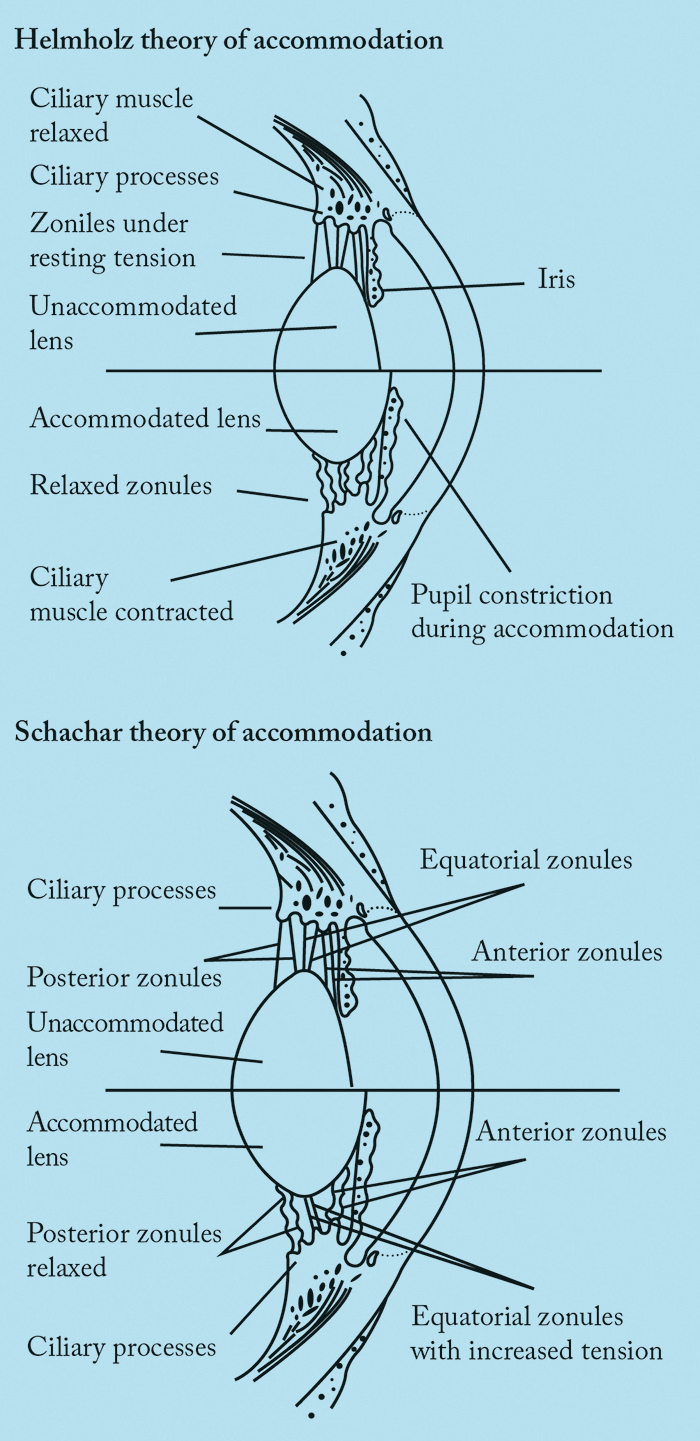
To appreciate why some pharmacological formulations may or may not work in presbyopia, it is essential to understand accommodation. Accommodation isn’t a completely settled topic: there are a number of competing theories that seek to explain the mechanism(s), with Hermann von Helmholtz’s 166 year-old theory being the most widely accepted. Von Helmholtz suggested that when the eye is at rest and focused for distance, the ciliary muscle is relaxed (Figure 1). To focus on a near object, the ciliary muscle contracts, causing the ciliary body to move forward and towards the axis of the eye. Simultaneously, the tension of the zonular fibers around the lens equator relaxes, which allows a soft lens to be molded by the elastic capsule into a more spherical and accommodative form. A larger, stiffer lens – such as one that arises from ageing – will not change shape as much, thereby compromising accommodation. Ronald Schachar proposed an alternative theory: that only the equatorial zonules are under tension during accommodation (Figure 1). When the ciliary muscle contracts, equatorial zonular tension is increased, causing the central surfaces of the crystalline lens to steepen, the central thickness of the lens to increase, and the peripheral surfaces of the lens to flatten. The increased equatorial zonular tension keeps the lens stable and flattens the peripheral lens surface during accommodation. In this scenario, presbyopia occurs because the equatorial diameter increases with age. However, this theory is opposed by the well-documented occurrence of lens stiffening and failure of scleral surgical approaches (which are based on Schachar’s theory) to correct presbyopia. Furthermore, studies have shown that it is axial thickness that is increased and not equatorial diameter. The most important aspect to remember is that true accommodation results in a dioptric change in the power of the eye. Most interventions achieve pseudoaccommodation: functional near vision from non-accommodative factors like small pupils, against-the-rule astigmatism and spherical aberration. Indeed, most pharmacological treatments attempt to exploit pseudoaccommodation from a small pupil.1. Muscarinic agonists with non-steroidal anti-inflammatory (NSAID) agents
Many groups have investigated this combination over the years (Table 2), the rationale being that a muscarinic agonist causes the ciliary muscle to contract (with a corresponding increase in lens thickness). However, this may only be achievable in younger presbyopes, as stiffer lenses are less easily induced into accommodation. Muscarinic agonists also cause miosis with resulting increase in depth of focus and pseudoaccommodation. What about the NSAIDs? Jorge Benozzi’s group has advocated the use of diclofenac for the inhibition of inflammation in the anterior uveal tract, claiming that it decreases spasmodic ciliary contraction, pigment dispersion and posterior synechia formation secondary to parasympathomimetic drugs (9). However, it must be noted that our group was unable to find any evidence to substantiate that NSAIDs achieve this, and pilocarpine is not known to cause pigment dispersion or posterior synechiae in normal eyes. Similarly, Patel et al. (10) have claimed that including NSAIDs with muscarinic agents prolongs the effects of the parasympathomimetic agent through the inhibition of prostaglandin synthesis in the anterior uvea. This seems counterintuitive – NSAIDs are used in cataract surgery to help prevent miosis – but nevertheless, Patel and Salamun believe it shows promise and have patented the approach along with Claes Feinbaum. They are currently attempting to minimize any adverse effects by using an intravitreal micro-insert to slowly release low concentrations of the insert’s ingredients. The micro-insert should have a number of advantages over repeated drop instillation: topical administration is associated with drug loss through the eye’s natural drainage channels, and this permits lower drug concentrations to be used. To date, no study data has been published on this device (10).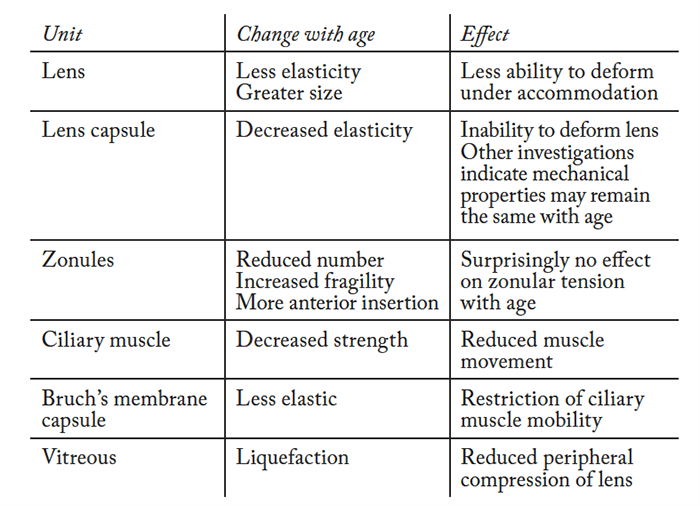
Juan-Carlos Abad (13) has attempted to exploit the pharmacology of NSAIDs one step further. NSAIDs inhibit the cyclooxygenase (COX) enzyme family, which are responsible for prostaglandin and thromboxane synthesis. Humans have two functional COX isoforms, COX-1 and COX-2, with COX-2 typically being expressed in inflamed tissue. Abad’s patent identifies using COX-2 specific inhibitors in combination with a cholinergic or muscarinic agent in an attempt to target COX-2 specific pro-inflammatory mediator production, but sparing COX-1’s “housekeeping” prostanoid production. Whether there is an advantage in this targeted approach over non-specific NSAIDS remains to be seen. A slightly different drug combination approach was taken by Humberto Carrera, who combined pilocarpine with the NSAID, bromfenac. His rationale was that bromfenac’s duration of action can be as long as 24 hours, and this should allow for a once-daily topical application – unlike diclofenac, which has an ocular half-life of under two hours. However, once again, no published studies are available on this formulation to date (14). We must note here that presbyopia is a benign condition, so it is essential not to advocate potentially iatrogenic therapies. NSAID drops such as diclofenac have been associated with devastating adverse effects, such as corneal melt, epithelial defect and sterile infiltrates, so extensive patient evaluation is required before long-term treatment initiation is warranted.
2. Muscarinic agonist with sympathetic agonist
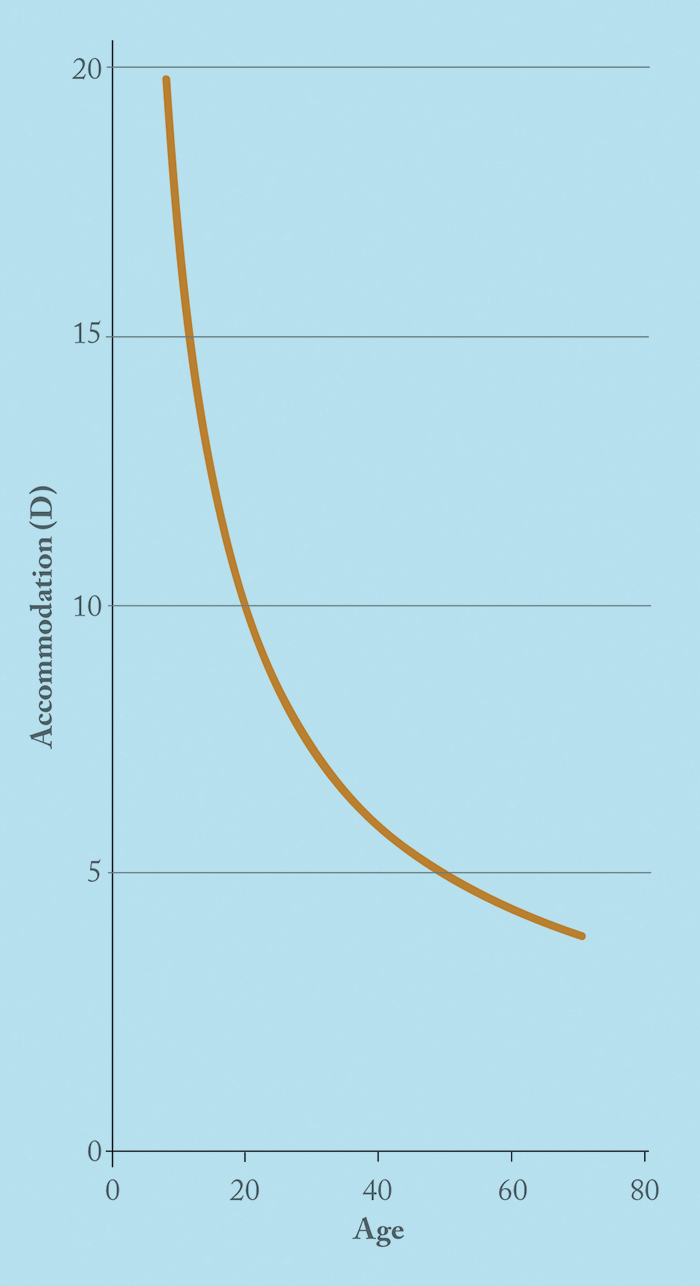
Carbachol is a parasympathomimetic agent and, unlike pilocarpine, is a full agonist that also promotes release of acetylcholine from parasympathetic nerve endings. Additionally, its carbamate structure means it may also inhibit cholinesterase enzymes (15). In terms of inducing miosis, the most commonly used strength of carbachol is 2.25% (which is equivalent in effect to about 3% pilocarpine; (16)). Brimonidine is an α2-receptor agonist, licensed in glaucoma, exhibits pupillary action, and can produce significant miosis, typically in low light conditions. Kaufman (18),(19) presented a study (summarized in Table 3) of the combination of both classes of drugs. Kaufman states “each combination was tested in each patient” but it is unclear if this was a true cross-over study or why uncorrected near visual acuity (UNVA) rather than best distance corrected near visual acuity (BDCNVA) was used. The near and distance visual acuities were measured 1, 2, 4, and 8 hours after instillation of the drugs. The results demonstrate the preparation is effective – but that adverse effects remain an issue. In what appears to be an extension of Kaufman’s work, Abdelkader (20) looked at carbachol 2.25% in combination with brimonidine 0.2%. The drops resulted in statistically significant UNVA improvements, and all patients stated they would continue to use the drops if available (whereas none were prepared to continue using the placebo drops). It’s well known from pilocarpine use in glaucoma that patients experience a dull headache on initiation with pilocarpine therapy, but that this should improve with time. The group have recently published another clinical trial (23), which compares the 3% carbachol formulation with brimonidine 0.2% in only 10 patients – but again, statistically significant results were shown. Given that they recruited almost five times as many patients for the 2.25% formulation (20), one can infer the formulation they likely favor. Interestingly, the trial was registered on November 11, 2016 but received by the journal in July 2016. Retrospective trial registration has been discouraged for some time by the ICJME (24). Allergic reactions to ophthalmic agents for the treatment of presbyopia have not been extensively studied. However, brimonidine (when used for the treatment of glaucoma), has. Blondeau (25) found that that up to 25.7 percent of patients with glaucoma experienced such a reaction in his study. Another combination of a muscarinic agonist with a sympathetic agent (pilocarpine and phenylephrine) has also been evaluated as a presbyopia therapy (21). Phenylephrine is a α1-adrenergic agent – but has shown little to no effect on the ciliary muscle or the centrally stimulated accommodative response. In the past, this combination was used as the Mapstone test, which consists of phenylephrine 10% and pilocarpine 2%, as provocation of closed-angle glaucoma. The proponent of this combination, Vejarano (22), has anecdotally stated that he has used the drops on himself for over five years with no change to his distant UCVA and a 2 line improvement in UNVA. Vejarano has not reported any adverse effects (such as brow ache or headache), but if pilocarpine does cause ciliary spasm leading to these adverse effects, they may remain unresolved by the addition of phenylephrine. It is also unclear how well patients tolerate the worsening of distance vision in the first hour of instillation, which has implications on, for example, operating heavy machinery and driving. The worsening may be caused by a delay in maximal pilocarpine action, resulting in an initial unopposed phenylephrine-induced mydriasis – the iris’ melanin acts as a pilocarpine reservoir, delaying it from working on the ciliary muscle immediately (26).A 45 year-old will have around 4 D of accommodative power but only uses around 2 D of this comfortably (Figure 2). The usual reading distance is around 40 cm and this requires 2.5 D of accommodation. Therefore a recovery of only 1–2 D would be sufficient to treat most presbyopic patients.
3. Muscarinic agonist with muscarinic antagonist
The combination of a muscarinic agonist with a muscarinic antagonist is found in PRX-100, a proprietary preparation developed by ‘Presbyopia Therapies’, which contains aceclidine and tropicamide. Aceclidine is a muscarinic agonist that is less potent than pilocarpine and carbachol; tropicamide is an anti-muscarinic agent. The combination is reported to effectively cause miosis without stimulating accommodation and, according to Presbyopia Therapies’ internal data, 1.6–1.9 mm is the optimum pupil diameter. Results reported in an article by Steven Dell are summarized in Table 4. Dell attributes the beneficial effects of PRX-100 under scotopic conditions to the reduction in light that’s received by retina, which is thanks to the smaller pupil diameter being offset by an improvement in contrast sensitivity and the elimination of stray light (27). However, this effect remains poorly understood: the amount of light entering is decreased, so presumably retinal adaptation is also occurring. This latter formulation perhaps has shown the most promise for patients given that it has progressed through various stages of regulatory approval. Indeed, a Phase II US clinical trial has been completed but (at the time of writing this article) is still unpublished (28).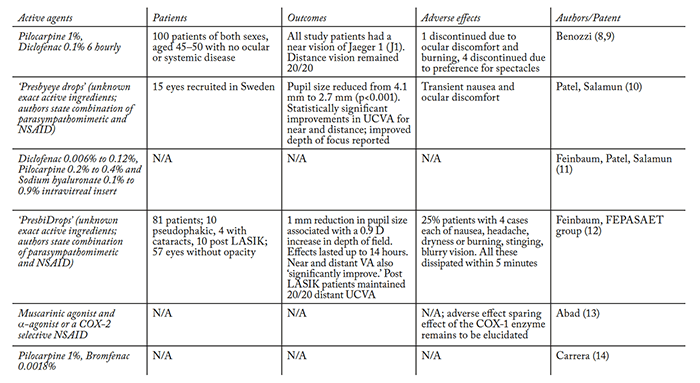
The mydriatic, tropicamide, has the opposite effect of aceclidine. Its use can appear counterintuitive, but a closer look at its pharmacology reveals why it may have been chosen. A study by German et al., (26) found that tropicamide displays a much higher affinity for iris M3 receptors (as opposed to ciliary M3 receptors) compared with other anti-muscarinic agents. What this allows is pupil dilation with minimal influence upon accommodation. This predilection for the iris may reduce the sphincter pupillae spasm with the minimal undesired antagonism of ciliary contraction, which is required for accommodation. In addition, its small effect on the ciliary muscle may explain why no brow or headache has been reported. The effect is well recognized and is exploited in the use of cyclopentolate, another muscarinic antagonist, to reduce aching pain caused by ciliary and iris spasm in corneal abrasion.
4. AGN-199201 and AGN-190584
AGN-199201 (presumed to be oxymetazoline, an α-sympathomimetic agent (29)) and AGN-190584 (a currently unknown agent) are two compounds, used together, currently under investigation by Allergan. The first of two Phase II clinical trials has been completed – and some results have been published (30). A second has been registered but, at the time of writing, patient recruitment has yet to begin (31). Oxymetazoline’s α-adrenergic action causes vasoconstriction, which is exploited for its use as a nasal decongestant and ocular anti-hyperemia agent. Its use is often restricted to several days due to rebound nasal congestion – an effect seen even with ocular use. However, α-receptor agonism in the eye acts on the iris dilator muscles to produce mydriasis – which is undesirable when you’re trying to treat presbyopia, as it decreases the depth of focus. It may be that AGN-199201 is only being included to attenuate an adverse effect caused by AGN-190584, such as hyperemia, or to allow AGN-190584 to remain in the eye longer and slow systemic absorption; it may even induce synergy. Allergan recruited 65 participants in a trial with the above mentioned agents (30), the results of which are summarized in Table 4. Combining AGN-190584 with oxymetazoline did not appear to negate the formers’ adverse effects. But the combination from Allergan has clearly shown some promise and are pursuing it in a second Phase II study.
5. Sympathetic antagonist with muscarinic agonist
It is possible that a muscarinic agonist and an α-sympathetic antagonist agent could act synergistically to allow miosis; not only would that assist in increasing pseudoaccommodation but would also reduce ciliary body spasm (because of a reduced muscarinic dose and possible opposing effects of the drugs at the ciliary muscle) and its putatively associated adverse effects, such as brow ache. The decrease in pupil size is the mechanism to increase the depth of focus and hence improve UNVA. It is a combination of drug classes that has been proposed by Anant Sharma in two formulations: pilocarpine with dapiprazole and pilocarpine with thymoxamine (33). Thymoxamine is a competitive post-junctional α1-antagonist that has been investigated for the discernment between angle-closure and open-angle glaucoma, and to reverse phenylephrine-induced pupil dilation. As sympathetic innervation has very little influence on the ciliary muscle (although opposite to muscarinic agonism), thymoxamine allows the size of the pupil to be affected without significantly affecting the ciliary muscle. The half-life is about 10 hours which is much longer than that of pilocarpine, which is about 1 hour (34). Susan Small and her colleagues showed back in 1976 that after 90 minutes, mean reduction in pupil size with thymoxamine was 1.6 mm with a 1 D increase in accommodation (35). The other proposed sympathetic antagonist, dapiprazole, has been studied for reversal of pharmacologically induced mydriasis and shares thymoxamine’s mechanism of action. When Wilcox (36) used dapiprazole 0.5%, the greatest increase in accommodation was gained with two drops as opposed to one drop. Notably, brown eyes were slower in their reversal of mydriasis. β-blockers, such as timolol, are another class of sympathetic agents that could be used. The iris and, to a lesser extent, the ciliary muscle also have β-adrenergic receptors, which when antagonized cause contraction, miosis and an increase in accommodation. However, timolol’s use may be limited as it can cause significant systemic adverse effects. Despite the drawbacks, Neufeld patented a preparation consisting of a β-blockers only (37), but no trials evaluating its effect on presbyopia have been performed.
Direct lens manipulation: softening
As noted earlier, perhaps the most significant hampering of accommodation occurs due to lens stiffening, which is caused by the crystalline protein’s sulfhydryl groups undergoing oxidation to disulfides as the human lens ages (38). Topically administered ester derivatives of lipoic acid have been investigated as lens softening agents. In fact, the choline ester of lipoid acid (LACE) was patented (as EV06) by Encore Vision for this purpose (39). LACE is a prodrug; it penetrates the cornea where it is quickly metabolized into choline and lipoic acid. Enzymes within the lens fiber cells chemically reduce the lipoic acid to dihydrolipoic acid, which is thought to reduce the disulfide bonds in the lens, restoring the lens’ “softness” – and, hopefully, natural accommodation. Encore Vision announced some (as yet) unpublished results from their Phase I/II randomized, double-masked, multicenter study, which examines the safety and efficacy of EV06/LACE 1.5% (compared with placebo) in 75 patients aged 45–55 years, over a 90 day period for the treatment of presbyopia and the primary endpoint of BDCNVA. In the EV06 group, mean change from baseline was a 0.191 LogMAR improvement and 0.095 LogMAR with placebo. The drops were “well tolerated and not associated with any significant adverse effects” (40). The group still needs to ascertain dosing frequency in a Phase III study. Initial results are encouraging and perhaps underline that lens stiffening plays a prominent part in the etiology of presbyopia – and this may explain Novartis’ announcement at the end of 2016 that they are to acquire Encore Vision (41).
Are we nearly there yet?
Pharmacological presbyopia therapy continues to evolve – but it hasn’t matured to a point where widespread adoption is on the horizon. Although there are data available on the various formulations mentioned in this article, they are often from unpublished studies and/or only available through news articles. The lack of peer-reviewed information – and a general absence of large-scale studies with robust data – makes appraisal of this approach to presbyopia therapy frustrating. It appears that there are only two approaches to the pharmacological treatment of presbyopia: generating a small pupil size, and lens softening. Any intervention that takes the first approach has to tick a number of boxes to be successful: it must be long-acting, produce significant miosis, have minimal or no myopic shift and be relatively side-effect free. To achieve this, it seems that the active ingredients must not only act synergistically but also allay any shortcomings (or side effects) of their counterparts; the combination of a muscarinic agonist and sympathetic antagonist appears to best create this synergy. We all know that we can increase the depth of focus by reducing aperture. Even monocular pharmacologic treatment with a single miotic agent has been shown to result in acceptable reading vision for many presbyopes, even in older recipients. And this same increase in the depth of defocus may improve distance vision in low hyperopes. It is also important to consider whether there would be an effect from other potential variables; for example, different colored irides and different ethnicities. Perhaps the most exciting formulations remain the lens softeners, which address presbyopia at a fundamental (and potentially longer-lasting) level. Although this is a much newer approach than the pharmacological induction of small aperture optics, safe and effective lens softening holds immense promise as a presbyopia intervention. Even if the accommodative outcomes and implications are currently difficult to predict, it’s still exciting times for topical ‘presbyopic’ treatments. Shafi Balal is a trainee doctor in North Central Thames London Deanery, UK. Raquel Gil-Cazorla is a clinical and research optometrist at Aston University, Birmingham, UK. Shehzad Naroo is a Reader at Aston University, UK. Anant Sharma is a consultant ophthalmic surgeon at Moorfields Eye hospital, Bedford, UK. Sunil Shah is a consultant ophthalmologist at the Birmingham and Midland Eye Centre, Birmingham, UK. Declaration Shafi Balal declares no conflict of interest. Shehzad Naroo has been awarded a research grant to investigate lens softening using femtosecond laser and a research grant to investigate scleral surgery to help presbyopia and grants to investigate intraocular lenses for presbyopic patients. Anant Sharma is an intellectual property (IP) holder of related technologies and products. Sunil Shah is a shareholder in company holding IP for presbyopia related technologies and products.References
- MA Croft, A Glasser, PL Kaufman, “Accommodation and presbyopia”, Int Ophthalmol Clin, 41, 33–46 (2001). PMID: 11290920. SA Naroo, “Is presbyopia the Holy Grail?”, College of Optometrists Annual Conference (2007). R Gil-Cazorla, S Shah, SA Naroo, “A review of the surgical options for the correction of presbyopia”, Br J Ophthalmol, 100, 62–70 (2016). PMID: 25908836. DA Atchison, “Accommodation and presbyopia”, Ophthalmic Physiol Opt, 15, 255–272 (1995). PMID: 7667018. T Kohnen, DD Koch, “Cataract and refractive surgery: progress III”, Springer Berlin Heidelberg; 2008. S Agarwal, A Agarwal, DJ Apple, “Textbook of Ophthalmology”, Jaypee Brothers (2002). A Duane, “Normal values of the accommodation at all ages”, JAMA, LIX, 1010–1013 (1912). JL Benozzi, “Ophthalmic compositions of parasympathetic stimulants and anti-inflammatories for use in the treatment of presbyopia” (2007). Patent publication number: WO2008075149 A2. Available at: bit.ly/Benozzi. Last accessed December 14, 2016. J Benozzi, G Benozzi, B Orman, “Presbyopia: a new potential pharmacological treatment”, Med Hypothesis Discov Innov Ophthalmol, 1, 3–5 (2012). PMID: 24600609. S Patel, K Matovic, “Pharmacological correction of presbyopia”, ESCRS Conference Paper (2013). CG Feinbaum, S Patel, F Šalamun, “Pharmacological ophthalmic composition for use in the correction of presbyopia and its administration” (2014). Patent publication number: WO2015122853 A1. Available at: http://bit.ly/feinbaum. Last accessed December 14, 2016. CG Krader, “Simple solution for presbyopia”, Ophthalmol. Times (2015). Available at: bit.ly/ss_presbyopia. Last accessed December 14, 2016. JC Abad, “Compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism” (2012). Patent publication number: WO2013041967 A2. Available at: bit.ly/abadpatent. Last accessed December 14, 2016. HJV Rodríguez, DH Carrera, “Composición oftálmica para la corrección de la presbicia” (2014). Patent publication number: WO2015092087A1. Available at: bit.ly/Rodríguez. Last accessed December 14, 2016. MA Croft et al., “Accommodation and ciliary muscle muscarinic receptors after echothiophate”, IOVS, 32, 3288–3297 (1991). D Pavan-Langston, “Manual of Ocular Diagnosis and Therapy”, Wolters Kluwer Health/Lippincott Williams & Wilkins (2008). HE Kaufman, “Preparations and methods for ameliorating or reducing presbyopia” (2010). Patent publication number: US8455494 B2. Available at: bit.ly/KaufmanHE. Last accessed December 14, 2016. H Larkin, “Eyedrops: monocular topical miotics may improve near vision without distant vision loss”, Eurotimes, 17 (2012). Available at: bit.ly/Larkin1. Last accessed December 14, 2016. S Kaufman, “Addressing presbyopia pharmacologically”, Ophthalmology Times (2012). Available at: bit.ly/ss_presbyopia3. Last accessed December 14, 2016. A Abdelkader, “Improved presbyopic vision with miotics”, Eye Contact Lens, 41, 323–327 (2015). PMID: 25806674. RLF Vejarano, “Ophthalmic formulation and method for ameliorating presbyopia” (2012). Patent publication number: US20140024642 A1. Available at: bit.ly/Vejarano. Last accessed December 14, 2016. CG Krader, “Topical drops show promise as treatment for presbyopia”, Ophthalmology Times (2016). Available at: bit.ly/ss_presbyopia2. Last accessed December 14, 2016. A Abdelkader, HE Kaufman, “Clinical outcomes of combined versus separate carbachol and brimonidine drops in correcting presbyopia”, Eye Vis, 3, 31 (2016). PMID: 27981057. SL Harriman, J Patel, “When are clinical trials registered? An analysis of prospective versus retrospective registration”, Trials, 17, 187 (2016). PMID: 27079379. P Blondeau, JA Rousseau, “Allergic reactions to brimonidine in patients treated for glaucoma”, Can J Ophthalmol, 37, 21–26 (2002). PMID: 11865954. EJ German, D Wood, MA Hurst, “Ocular effects of antimuscarinic compounds: is clinical effect determined by binding affinity for muscarinic receptors or melanin pigment?”, J Ocul Pharmacol Ther, 15, 257–269 (1999). PMID: 10385135. SJ Dell, “Eye drop may provide a pharmacological treatment for presbyopia”, www.healio.com (2014). Available at: bit.ly/sjdell01. Last accessed December 14, 2016. Presbyopia Therapies, “Evaluation of the efficacy and safety of PRX-100 in the treatment of early to moderate presbyopia”, NCT02554396, ClinicalTrials.gov (2016). Available at: bit.ly/NCT02554396. Last accessed December 14, 2016. FA Moustafa, LF Sandoval, SR Feldman, “Rosacea: new and emerging treatments”, Drugs, 74, 1457–1465 (2014). PMID: 25154627. Allergan, “Safety and efficacy of AGN-199201 and AGN-190584 in patients with presbyopia”, NCT02197806, ClinicalTrials.gov (2014). Available at: bit.ly/NCT02197806. Last accessed December 14, 2016. Allergan, “A study of the concurrent use of AGN-190584 and AGN-199201 in participants with presbyopia”, ClinicalTrials.gov Identifier: NCT02595528 (2016). Available at: bit.ly/NCT02595528. Last accessed December 14, 2016. G Horn, L Nordan, “Compositions and methods for the treatment of presbyopia” (2014). Patent publication number: US9089562 B2. Available at: bit.ly/horn-nordan. Last accessed December 20, 2016. A Sharma, “Optical correction” (2008). Patent publication number: US20090156606 A1 (2009). Available at: bit.ly/sharmacorrection. Last accessed December 14, 2016. Pilocarpine hydrochloride (solution, ophthalmic) monograph (2009). Available from: bit.ly/pilocarpinemono. S Small, JH Stewart-Jones, “Influence of thymoxamine on changes in pupil diameter and accommodation produced by homatropine and ephedrine”, Br J Ophthalmol, 60, 132–134 (1976). PMID: 131576. CS Wilcox, et al., “Comparison of the effects on pupil size and accommodation of three regimens of topical dapiprazole”, Br J Ophthalmol, 79, 544–548 (1995). PMID: 7626570. AH Neufeld, “Methods and products for treating presbyopia”, Filing date: Nov 1993. Publishing date: January 1996. US5488050A (1996). L Takemoto, “Increase in the intramolecular disulfide bonding of alpha-a crystallin during aging of the human lens”, Exp Eye Res 63, 585–590 (1996). PMID: 8994362. EncoreHealth LLC, “Choline esters” (2014). Patent publication number: US9326970 B2. Available at: bit.ly/cholineesters. Last accessed December 14, 2016. B Burns, “Encore Vision Reports Positive Phase I/II Results” (2016). Available at: http://bit.ly/EV06presbyopia. Last accessed December 14, 2016. Novartis AG, “Novartis bolsters ophthalmology pipeline though acquisition of Encore Vision, Inc” (2016). Available at: bit.ly/encorenovartis. Last accessed December 20, 2016.
