The Innovation Awards 2017
The field and practice of ophthalmology is constantly being shaped by the driving force of innovation. As one of the most innovative fields in medicine, ophthalmology is often at the forefront of cutting-edge technologies and treatments. Here, some of the leading innovators from the ophthalmic space present their latest and greatest offerings: from imagers and diagnostics, to cutting edge refractive surgery and glaucoma care.
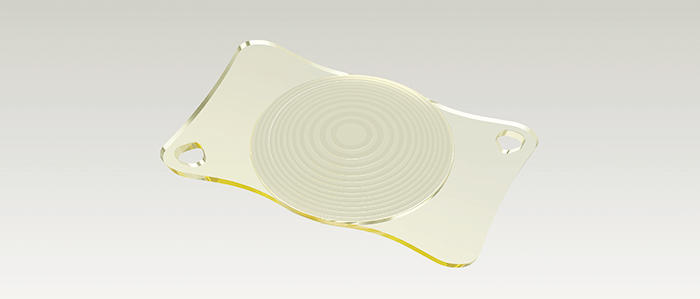
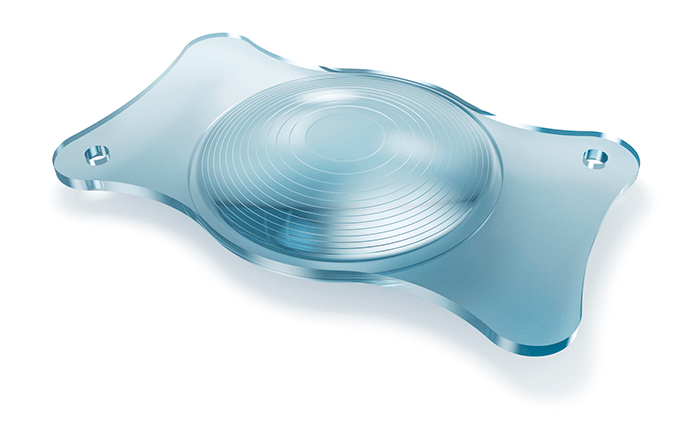
‘Premium’ patients expect premium outcomes. When it comes to multifocal IOLs, it’s the surgeon’s job to give their patients what they’ve paid for. Both surgeon and patient, therefore, require a premium IOL that can meet the demands (and the high expectations) that are inherent in premium cataract and refractive lens exchange procedures. Multifocal IOLs – and in particular trifocal IOLs – provide patients with good visual acuity at all distances. But this has come at a cost: the optical compromises inherent in such lens designs can lead to visual side effects like halo and glare. If patients can’t tolerate them, the alternative is often placing a monofocal lens. These are safe in terms of visual side-effects, but come with a glaring drawback: patients need to wear spectacles for near and intermediate vision. Extended depth-of-focus (EDoF) IOLs provide an excellent compromise of the benefits of both monofocal and multifocal IOLs: more spectacle independence than monofocal IOLs, with fewer visual side effects than multifocal IOLs. If you have a patient who leads an active lifestyle, who wants to be largely spectacle-independent, who may be particularly sensitive to visual side effects, and who is happy to occasionally wear reading glasses, EDoF lenses could be a great choice. However, such lens designs still involve optical compromises and visual side effects – meaning there’s still room for improvement. It has taken real innovation from ZEISS, including simulations with about 50,000 optical design candidates, to push forward the field of EDoF IOLs: the result is a lens that provides the widest range of focus of any lens of its type on the market today – and simultaneously minimizing visual side effects and contrast loss. That lens is the AT LARA 829MP, with each letter in ‘LARA’ representing an optical innovation by ZEISS: L – “Light Bridge” optical design, providing the widest range of focus among EDoF IOLs. A – Aspheric optics that are biometrically optimized and neutral. The aberration neutral aspheric design supports depth of focus and post-LASIK usage. R – Reduced visual side effects, thanks to patented Smooth Micro Phase (SMP) technology and an EDoF design that results in fewer visual side effects than multifocal IOLs. A – Advanced chromatic optics, with a color-optimized optical design for increased contrast sensitivity. The Light Bridge optical design is based on a unique diffractive optic with two focus additions, creating a continuous focus extension that allows patients to see sharply without visual aid at distances relevant to most of their daily activities. AT LARA 829MP’s aberration-neutral aspheric design and advanced chromatic correction allow for optimized contrast sensitivity. ZEISS’ SMP technology combines a diffractive optical design with a unique transition between optical powers. This design allows for an IOL surface profile that can be manufactured more precisely thus reducing light scatter inherent in other IOLs. As a consequence, a smaller fraction of the incident light is misdirected, and accordingly, minimizes visual side effects. Not only does the AT LARA 829MP represent an innovation in optics – it’s also an innovation in manufacturing. With AT LARA 829MP, ophthalmic surgeons can decrease spectacle dependence for a broader group of patients and address the growing need for improved intermediate vision performance, which is important for activities such as working at a computer. ZEISS AT LARA EDoF IOL enhances the range of vision for patients by creating an elongated focus range. Now, doctors have a new option to provide superior visual outcomes for cataract patients for whom multifocal IOLs might not be the optimal choice because of sensitivity to visual side effects, such as halo and glare at night.
Florian Kretz, Chief Executive Officer and Lead Surgeon at Augenärzte Gerl, Kretz & Kollegen in Rheine, Germany, says, “AT LARA from ZEISS offers us an additional option for individualized patient care. It enhances the intermediate visual acuity and offers reduced optical phenomena with increased optical performance for distance and intermediate range.”
Balasubramaniam Ilango, Medical Director of OPTIMAX clinics, U.K. “My patients are delighted and very happy with AT LARA 829MP. Their visual acuity is excellent over a wide range of distances and so far they do not report about any visual side effects.”
Mild to moderate POAG, pseudoexfoliative glaucoma or pigmentary glaucoma
- C/D ratio ≤0.8
- pre-op IOP up to 30 mmHg (medicated)
- target post-op IOP to 15 mmHg
Glaukos has been at the forefront of glaucoma management since 1998, and has built an unparalleled reputation for innovations that are progressively transforming the treatment of this most problematic disease. Now, the company that pioneered MIGS by introducing the iStent® – previously the smallest medical implant in standard use – has further enhanced its revolutionary product by developing the iStent inject® device, which is smaller still. The original iStent’s advantages are well-known; the creation of a permanent trabecular micro-bypass allows the resumption of fluid flow out of the eye, thereby reducing IOP and bringing potential benefits to patients such as delayed disease progression and avoiding the need for complex, invasive surgery. In addition, the IOP reduction achieved is often sufficient for iStent® recipients to reduce or entirely eliminate the use of eye-drops. And given glaucoma medication issues – namely, uncomfortable side-effects, poor compliance and cost – this represents a real benefit of the iStent® system. So what’s new about the iStent inject® system? iStent inject® features heparin-coated titanium microstents with a central inlet and four outlets to optimize flow and collector channel access – like iStent®, only smaller. The delivery device comprises a neat, hand-held injector – a cam-driven, single-use sterile unit, with a window in the insertion tube to optimize visualization of stent delivery during implantation. Furthermore, the iStent inject® device is preloaded with not one but two stents, and the innovative design of the injector delivers both stents into the trabecular meshwork. Therefore, as well as providing a convenient and cost-effective means of stent insertion, the iStent inject® system allows the surgeon to place two stents into trabecular meshwork in a single procedure – that is, via a single corneal entry point. The result? Further pressure reduction for minimal trauma: the safety profile is similar to that of cataract surgery alone (1), and the burden of patient follow-up after the iStent inject® procedure is no different from the follow-up associated with standard cataract procedures. Furthermore, and very encouragingly, the iStent inject® -associated reduction in medication needs is excellent; 72 percent of patients treated with iStent inject® no longer require glaucoma medication 12 months after the procedure (2). Finally, mindful of the real-life needs of ophthalmic surgeons, Glaukos has made the device applicable both in combination with cataract surgery and in standalone procedures. What next? The iStent® was already known as not only the first MIGS system but also as one of the ‘true’ ab interno MIGS systems. Now, by coupling two stents with the simple and novel iStent inject® implantation system, Glaukos is set to further increase uptake of the iStent inject® throughout the EU (acceptance is already impressive – over 300,000 patients have now received the iStent® or iStent inject®implants). The iStent inject® device is the latest example of how Glaukos is disrupting the traditional glaucoma management paradigm and revolutionizing treatment in this field – but it won’t be the last. The company has a suite of other products and indications in the pipeline (over 20 company-sponsored clinical trials in progress), and has ambitious plans for the new iDoseTM device (a forthcoming product for continuous intra-ocular elution of medication over extended periods). In the meantime, surgeons caring for the extensive group of patients likely to benefit from the iStent® (see ‘eligible patients’), now have an additional tool in the form of the iStent inject®, which allows them to offer patients more therapeutic impact from a single procedure. References
- TH Neuhann et al, “Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: Long-term results”, J Cataract Refract Surg, 41, 2664-2671 (2015). PMID: 26796447
- L Voskanyan et al., “Prospective unmasked evaluation of the iStent Inject system for open-angle glaucoma: Synergy trial,” Adv Ther, 31, 189–201 (2014). PMID: 24452726.
AMD remains the leading cause of adult blindness – a result not only of limited treatment options but also diagnostic failings. Even experienced ophthalmologists miss 25 percent of AMD cases (1), meaning that diagnosis often is made only after irreversible visual acuity loss. Indeed, 78 percent of patients exhibit substantial vision loss at first treatment, and 37 percent are effectively blind in one eye. How can we address the problem? The obvious solution is to improve our diagnostic capabilities. Identifying AMD in its early stages would allow treatment of AMD before it causes irreversible damage. For example, AREDS2 nutritional supplements can reduce disease progression by 30 percent. This early diagnosis–proactive treatment paradigm is the logic behind MacuLogix’ AdaptDx system. AdaptDx is a novel automated dark adaptometer, similar in operation to visual field analyzers used in glaucoma. Without any need for pre-adaptation or dilation, the device induces photobleaching by a brief, non-irritating flash; immediately afterwards, it measures the Rod Intercept (RI) – the time for the eye to adapt from bright light to darkness at a standard threshold stimulus. “RI is 90 percent AMD-specific and sensitive,” states Gregory Jackson, PhD (Chief Technology Officer). AdaptDx enables AMD diagnosis at least three years before it becomes apparent in structural exams, which, in turn, allows monitoring and early treatment to delay disease progression and preserve vision. With FDA clearance, Canadian clearance and CE marking for use in the EU, AdaptDx is now broadly available for clinical use. Ideally, it should be used to screen all patients over age 50, especially those with night vision complaints. Such screening would both uncover the true prevalence and – as more patients are diagnosed at earlier stages – provide motivation to develop better treatments for early and intermediate AMD. As William McPhee (CEO) summarizes: “Our goal is to eliminate blindness caused by AMD by changing the trajectory of AMD diagnosis, management and treatment with the AdaptDx.” Reference
- DC Neely, et al., “Prevalence of Undiagnosed Age-Related Macular Degeneration in Primary Eye Care”, JAMA Ophthalmol., 135, 570-575 (2017). PMID: 28448669.

Capsulotomy is the heart of cataract surgery – get it right and the rest follows. For the last 25 years, the standard of care has been continuous curvilinear capsulorhexis (CCC). Though developments in femtosecond laser technology have aimed to replace the manual rhexis procedure, there are cost factors and difficulties associated with the procedure. Mynosys co-founders David Sretavan and Chris Keller realized that the world did not need another capsulotomy device, it needed a better one – a device that would enhance patient outcomes with premium IOLs. The goal led to their Precision Pulse Capsulotomy concept – and the Zepto Automated Capsulotomy Device was born. The innovative and disruptive device creates anterior capsulotomies precisely on the patient’s visual axis: a 360 degree capsulotomy can be created in 4 milliseconds, and the resulting edges are stronger than those created by CCC or femtosecond laser. One of the key milestones in the company’s history was finding the perfect blend of suction, energy pulse and nitinol for the capsule suction tip. With a CE mark, FDA clearance and a successful FDA clinical trial under its belt, what’s next for Zepto? Mynosys plan to continue innovating in capsulotomy by developing devices of different sizes and for new uses, such as pediatrics. This device has a CE Mark and FDA clearance.
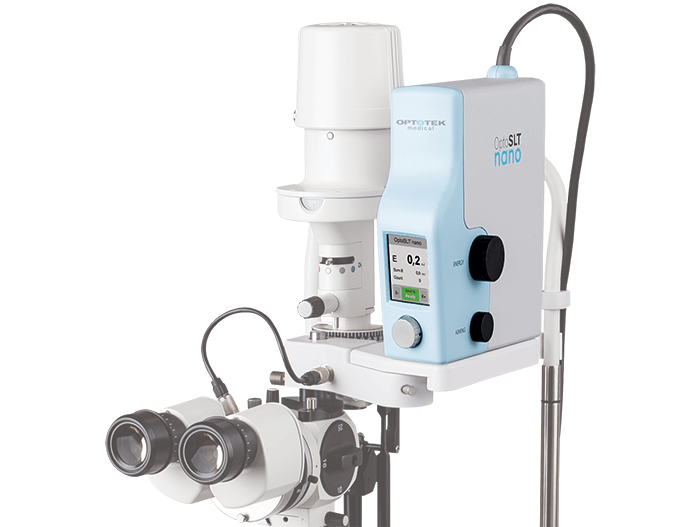
Selective laser trabeculoplasty (SLT) is a standard option for reducing intraocular pressure in glaucoma patients. But this decades-old technique has been transformed by a device that promises safer and faster procedures: OptoSLT nano, the first diode-pumped solid-state laser for SLT. Why the excitement? First, the OptoSLT nano provides significantly lower pulse variability (± 2.5%) than existing laser devices. One consequence of this is increased pulse speed (up to 5 Hz); another is enhanced safety. Both follow from the highly repeatable pulse energy delivered during treatment – as Andrej Vrečko (Optotek’s R&D Manager) says, “OptoSLT pulse energies are an order of magnitude more stable than competing systems.” Second, the OptoSLT nano is highly convenient: it is small, portable and can be used either as a complete device – with its own lifting mechanism and slit lamp – or can be used to upgrade most Zeiss and Haag Streit-type slit lamps. Adopting the OptoSLT nano does not require disruptive changes to the clinic. Finally, multiple studies have confirmed the clinical effectiveness of one nanosecond laser SLT. The Optotek device is now CE-approved, so ophthalmologists keen to prevent vision loss in their patients now have a faster, safer and more convenient option. “OptoSLT nano will make SLT the first-choice procedure for primary open angle glaucoma cases,” concludes Vrečko.
Not all multifocal IOLs are equal. A crucial part of the conversation between a patient who wants a multifocal IOL and the surgeon is finding out the patient’s ideal near and intermediate distances are – and then the multifocal IOL that would most closely match their preferences is selected. But the point is, vision isn’t just about near, intermediate and distance – it’s everything in between too. Let’s examine conventional trifocal IOLs that achieve multifocality through multiple diffractive rings with sharp edges. The sharp edges enable diffraction – they abrupt change in the path of light, after all – but they also lead to unwanted light scattering, which patients typically experience as glare and halo. Further, this light dispersion leads to light loss of up to 15 percent, which decreases patients’ contrast sensitivity too. Then there’s the elephant in the room: these lenses might offer patients near, intermediate and distance vision – but everything’s blurred in between. The answer is VSY Biotechnology’s patent-pending Sinusoidal Seamless Vision Technology (SVT). Unlike traditional trifocals, the only SVT trifocal IOL, the AcrivaUD Trinova has twelve unique sinusoidal stepless ridges. The smoothly varying surface profiles give rise not only to the highest levels of light transmission to the retina in the market (92 percent), but also optimum light distribution through all optical diameters. The result? Excellent modulation transfer function (MTF) at all distances, even in mesopic conditions, as well as excellent contrast sensitivity, reduced glare and halo, and outstanding dynamic visual performance – After all, the lens provides the widest depth of focus of all the currently available trifocal IOLs thanks to its EDOF feature. Furthermore, SVT also allows the AcrivaUD Trinova to compensate for minor post-op refractive errors and slight tilts. Ophthalmologists absolutely want to eliminate light loss (and the resulting loss in contrast sensitivity), glare, halo, and post-op refractive errors. The AcrivaUD Trinova lens has been commercially available since October 2017 and has been helping patients see better: near, intermediate and far – and everything in between, too.