
Contains promotional information by Alimera Sciences. Please click here to view the prescribing information and adverse event reporting information for ILUVIEN® (fluocinolone acetonide [FAc]).
National Institute for Health and Care Excellence (NICE) technology appraisals review the clinical and economic evidence for new and existing medicines and treatments. NICE has issued guidance on four pharmacological treatments for diabetic macular edema (DME): Lucentis® (ranibizumab, Novartis Pharmaceuticals Ltd.), Eylea® (aflibercept, Bayer Healthcare Ltd.), ILUVIEN® (fluocinolone acetonide [FAc], Alimera Sciences Ltd.) and OZURDEX® (dexamethasone, Allergan Pharmaceuticals Ireland).1–4 This article summarizes the NICE guidance and considers the cost-effectiveness of these treatments in different subsets of patients with DME: those who have a central retinal thickness (CRT) ≥400 µm and those who have a CRT <400 µm (74% of DME patients are estimated to fall in this latter group).5 The prescribing options currently used in clinical practice for patients who have a CRT <400 µm include bevacizumab (outside of its marketing authorization), laser photocoagulation,2,4 and a 'watch‑and‑wait' approach until CRT reaches 400 µm, when a therapy recommended for patients with DME who have a CRT ≥400 µm is commenced.2 NICE did not recommend ranibizumab, aflibercept, or dexamethasone for patients with a CRT <400 µm.1,2,4 In contrast, NICE did not use CRT as a criterion to define a patient population recommended for treatment with FAc,3 implying that the FAc implant can be used irrespective of CRT.
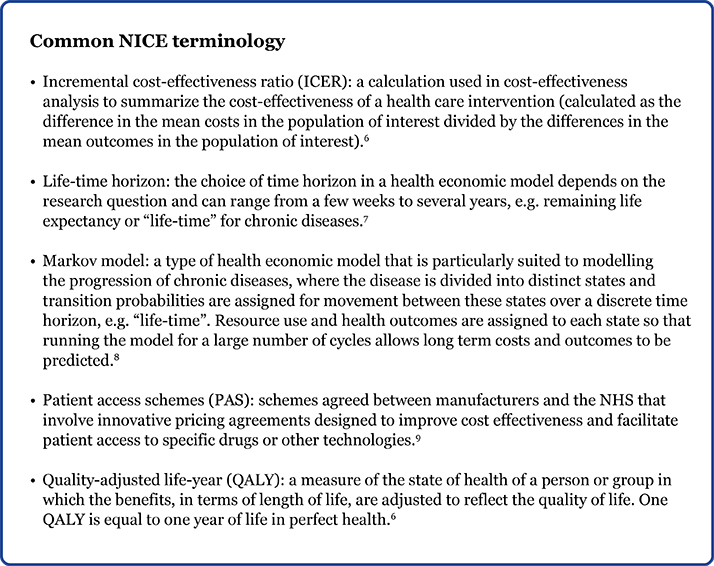
NICE guidance for DME Guidance based on the clinical and economic evidence for use of ranibizumab (technology appraisal [TA] 274), aflibercept (TA346), FAc (TA301) and dexamethasone (TA349) have been published online and are summarized in Table 1.
Table 1. NICE guidance for pharmacological treatment options for DME.1-4
The cost-effectiveness of ranibizumab, aflibercept, FAc and dexamethasone was determined independently using Markov-based models projected over 15 years (lifetime horizon for aflibercept), which captured costs for treatments, monitoring and adverse events (AEs), and those associated with blindness. Patient access schemes (PAS—schemes that involve innovative pricing agreements designed to improve cost-effectiveness and facilitate patient access to specific drugs or other technologies) were incorporated in incremental cost-effectiveness ratio (ICER) calculations and formed part of the deliberations for all products except dexamethasone. For each therapy, the cost-effectiveness is described or defined in quality-adjusted life-years (QALY) gained. If a QALY lies between £20,000 and £30,000, it is considered to be cost-effective. Anything above this range is not considered to be an effective use of NHS resources. The cost-effectiveness may also be compared with existing therapies and, in such cases, the most cost-effective therapy will be recommended. The cost-effectiveness of ranibizumab, aflibercept, the FAc implant and dexamethasone are reported individually below, highlighting the guidance for patients with DME who have a CRT ≥400 µm.
Ranibizumab Ranibizumab monotherapy was assessed in comparison with ranibizumab plus laser therapy or laser monotherapy for the treatment of DME in one eye (baseline best-corrected visual acuity [BCVA] of ≤75 letters).1 NICE “could not recommend ranibizumab as an effective use of NHS resources for the treatment of all people with DME”, as it considered “the most plausible ICER was likely to be above £30,000 per quality-adjusted life-year (QALY) gained”. However, NICE recommended the use of ranibizumab for patients with a “central retinal thickness (CRT) of 400 µm or more”, as it concluded the ICER would be under £25,000 per QALY gained. For further information on this guidance, please see www.nice.org.uk/guidance/ta274/.1

Aflibercept Aflibercept monotherapy was assessed in comparison with laser photocoagulation, bevacizumab, FAc, ranibizumab or dexamethasone for the treatment of people with DME with a baseline BCVA ≤75 letters.2 Participants were treated in either their study eye or fellow eye.2 NICE concluded that aflibercept was not a cost-effective use of NHS resources, compared with laser, for the treatment of all people with DME considered in the analysis. However, it concluded that aflibercept was cost-effective in the subgroup of people with DME and CRT ≥400 µm, where the ICER for aflibercept compared with laser was £21,958. It was noted that “the main comparator for this subgroup was ranibizumab”, but “no comparison with ranibizumab was included in the cost-effectiveness evidence for CRT subgroups”. There was, “no significant difference in clinical effectiveness” between aflibercept and ranibizumab, and “the ICER showed that aflibercept was cost-effective compared with ranibizumab” (ICER <£20,000 per QALY gained). For further information on this guidance, please see http://www.nice.org.uk/guidance/ta346.2

Fluocinolone acetonide The FAc intravitreal implant was assessed in comparison with optimized standard of care or laser photocoagulation monotherapy in people with visual impairment due to chronic pseudophakic DME after an inadequate response to prior therapy.3 Based on existing NICE guidance and clinical practice, the patient population considered suitable for the FAc intravitreal implant is that of patients who have had an insufficient response to laser photocoagulation and anti-VEGF therapies. Furthermore, for all patients with chronic DME considered in the analysis, ICERs ranged from £37,600 to £63,500 per QALY gained. For the pseudophakic subgroup, ICERs ranged from £17,500 to £30,000 per QALY gained. (NB: these ICERs take account of a PAS) NICE, therefore, only recommended the FAc intravitreal implant as a treatment option for patients with chronic DME in a pseudophakic eye that is insufficiently responsive to available therapies. For further information on this guidance, please see www.nice.org.uk/guidance/ta301.3

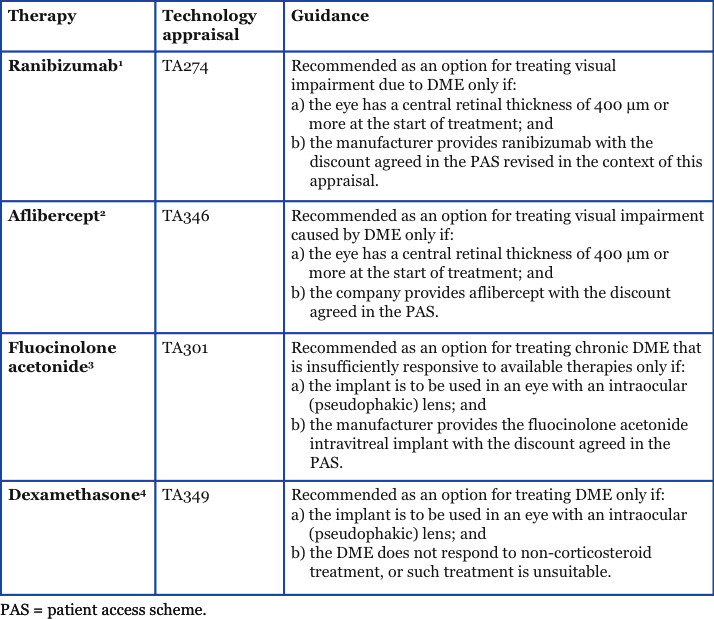
Update: Evidence for effectiveness of FAc in patients with a CRT <400 µm The implication that the FAc implant can be used irrespective of CRT is supported by the effectiveness of the FAc implant for the treatment of DME demonstrated in the Fluocinolone Acetonide for Diabetic Macular Edema (FAME) clinical trials. Participants in the trials were adults with DME whose CRT was ≥250 µm at baseline.10 New subgroup analysis of the FAME studies (from data on file) shows the effectiveness of the FAc implant in patients with chronic DME (≥ 3 years) and a CRT <400 µm versus patients with chronic DME and a CRT ≥400 µm (Figure 1).11 Changes in visual acuity were reported as the percentage of patients with chronic DME who achieved ≥15 letters from the baseline, and mean change in BCVA from the baseline. For both measures, the change in visual acuity in chronic DME patients treated with FAc versus the control group was similar in both the CRT <400 µm subgroup and the CRT ≥400 µm subgroup (Figure 1). Interestingly, in the published subgroup analysis from the FAME study of patients with chronic DME, the percentage of patients who achieved a gain in BCVA of ≥15 letters from the baseline after 36 months was 34.0%.10 This is similar to the percentage of chronic patients with a CRT <400 µm achieving the same outcome in the new data presented here (34.2%).11 Overall, these results suggest that the FAc implant is equally efficacious in patients with chronic DME regardless of CRT. The cost-effectiveness of treating DME patients with a CRT <400 µm with the FAc implant has also been explored and was based on the same assumptions as TA301—in terms of utility sources, the utility adjustment for the worse seeing eye, the treatment of the best and worst seeing eye in clinical practice, and responder split—but looking at DME irrespective of lens type (i.e. patients with phakic and pseudophakic lenses were included). Nearly all outcomes had a QALY below £30,000 with the discount agreed in the existing PAS.11 These data would suggest the FAc implant is cost-effective in this particular group and potentially could be judged as an effective use of NHS resources.
Figure 1. Changes in visual acuity in chronic DME patients based on starting central retinal thickness <400 µm (left panels) and ≥400 µm (right panels).11
* = p<0.05, paired comparisons between the control and 0.2 µg/d FAc.
Dexamethasone The dexamethasone intravitreal implant was assessed in comparison with a range of comparators for the treatment of people with visual impairment due to DME.4 Four scenarios were explored:
- Scenario 1: For people with a pseudophakic lens and a CRT ≥400 µm, it was concluded that if the confidential PAS for ranibizumab was included, dexamethasone was not recommended for use because of its lower QALY gain with a marginal difference in costs.
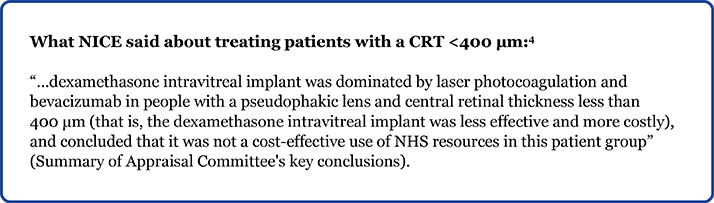
- Scenario 2: For people with a pseudophakic lens with a CRT <400 µm, it was noted that the dexamethasone intravitreal implant was dominated by laser photocoagulation therapy and bevacizumab.
- Scenario 3: For people without a pseudophakic lens and with DME unsuitable for or insufficiently responsive to non-corticosteroid therapy, it was “considered that the true value of the ICER would be greater than the ERG's (Evidence Review Group) new exploratory base-case ICER of £127,645 per QALY gained” and was, therefore, not recommended.
- Scenario 4: For people with a pseudophakic lens with DME that is unsuitable for or insufficiently responsive to non-corticosteroid therapy, it was noted that when the exact discount agreed in the PAS for the FAc intravitreal implant was taken into account, there was little difference in total costs and total QALYs of the FAc intravitreal implant and dexamethasone intravitreal implant. Therefore, the cost-effectiveness of the dexamethasone intravitreal implant was considered to be likely similar to the fluocinolone acetonide intravitreal implant.
Health economic uncertainties It should be noted that all interventions were assessed individually as part of a NICE single technology appraisal.1–4 All submitted economic analyses had limitations; common to all were limitations regarding the choice of utility estimates for the DME population. Other uncertainties included the assumptions and approaches for treatment in both eyes, applications to the cost of blindness, costs of hospital attendances and assumptions on moving from one health state to another. As previously noted, the availability of ranibizumab, aflibercept, and the FAc intravitreal implant is contingent on provision at the discount agreed in the respective patient access schemes.1–3

Dr Tejal Patel graduated from Brighton & Sussex Medical School in 2011. She has worked as an ophthalmology trainee at James Paget University Hospitals NHS Foundation Trust since 2013. She is actively involved in clinical research and teaches medical students at the University of East Anglia.

Look out for more DME content developed by Alimera Sciences on this website. We hope it supports your knowledge of DME and ILUVIEN, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.
REFERENCES
- National Institute for Health and Care Excellence, “Ranibizumab for treating diabetic macular oedema (rapid review of technology appraisal guidance 237). NICE technology appraisal guidance 274”, (2013). Available at: www.nice.org.uk/guidance/ta274/. Accessed Jan 5, 2016.
- National Institute for Health and Care Excellence, “Aflibercept for treating diabetic macular oedema. NICE technology appraisal guidance 346”, (2015). Available at: www.nice.org.uk/guidance/ta346. Accessed Jan 5, 2016.
- National Institute for Health and Care Excellence, “Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy (rapid review of technology appraisal guidance 271). NICE technology appraisal guidance 301”, (2013). Available at: www.nice.org.uk/guidance/ta301. Accessed Jan 5, 2016.
- National Institute for Health and Care Excellence, “Dexamethasone intravitreal implant for treating diabetic macular oedema. NICE technology appraisal guidance 349”, (2015). Available at: www.nice.org.uk/guidance/ta349. Accessed Jan 5, 2016.
- Diabetic Retinopathy Clinical Research Network. The relationship between OCT-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007; 114(3): 525–536.
- National Institute for Health and Care Excellence, Glossary. Available at: www.nice.org.uk/Glossary. Accessed Jan 5, 2016.
- Institute for Pharmacoeconomic Research, Vienna, “Guidelines on Health Economic Evaluation: Consensus paper”, (2006).
- A Briggs and M Sculpher, “An Introduction to Markov Modelling for Economic Evaluation”, Pharmacoeconomics 1998; 13(4), 397–409.
- National Institute for Health and Care Excellence, Patient access schemes liaison unit. Available at: www.nice.org.uk/about/what-we-do/patient-access-schemes-liaison-unit. Accessed Jan 5, 2016.
- P Campochiaro et al., “Sustained Delivery Fluocinolone Acetonide Vitreous Inserts Provide Benefit for at Least 3 Years in Patients with Diabetic Macular Edema”, Ophthalmology 2012; 119(10), 2125–2132. PMID: 22727177.
- Data on file. Alimera Sciences Limited.
- International Diabetes Federation. IDF Diabetes, 7 ed. Brussels, Belgium: International Diabetes Federation, 2015. Available at: www.diabetesatlas.org. Accessed March 17, 2016.
- T Patel, C Goldsmith, M Raja, “Case series evaluating intravitreal fluocinolone implant (ILUVIEN®; [Fluocinolone Acetonide; FAc]) in the treatment of patients with chronic diabetic macular edema (DME) insufficiently responsive to current treatment options”, presented at the 25th Meeting of the European Association for the Study of Diabetes Eye Complications Study Group (EASDec), 26–28 June, 2015, Turin, Italy (2015).
- I Klassen et al., “Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions”, Prog Retin Eye Res 2013; 34, 19–48. PMID: 23416119.
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal diseases, such as diabetic macular edema (DME), dry age-related macular degeneration (AMD), and retinal vein occlusion. In 2015, Alimera Sciences partnered with The Ophthalmologist to facilitate the publication of independently created educational content surrounding DME, a serious retinal complication associated with diabetes, which is increasing in incidence with the increasing prevalence of diabetes worldwide. Published content will include articles ranging from basic science and disease processes to overviews of clinical data, different surgical procedures, comparisons of treatment options, and practical advice for managing diabetic patients. With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend to provide educationally focused content to healthcare professionals across a wide range of topics in DME in order to both increase disease awareness and understanding, and to help improve patient outcomes. UK-ILV-MMM-0355 Date of preparation: August 2015 enquiries@alimerasciences.com

